Portal:Chemistry
2008/9 Schools Wikipedia Selection. Related subjects: Portals; Science
|
Welcome to the chemistry portal. Chemistry, from Greek language χυμεία meaning "study of liquids", is a branch of science. Modern chemistry focuses on the study of elements of the world and the bonds between elements. Chemistry also deals with composition, structure, and properties of substances and the transformations that they undergo. In the study of matter, chemistry also investigates its interactions with energy and itself. Because of the diversity of matter, which is mostly in the form of compounds, chemists often study how atoms of different chemical elements interact to form molecules, and how molecules interact with each other.
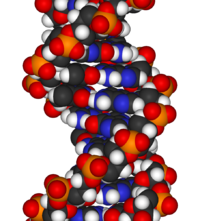
Deoxyribonucleic acid (DNA) is a nucleic acid that contains the genetic instructions for the development and function of living organisms. All living things contain DNA, with the exception of some viruses with RNA genomes. The main role of DNA in the cell is the long term storage of information. It is often compared to a blueprint, since it contains the instructions to construct other components of the cell, such as proteins and RNA molecules. The DNA segments that carry genetic information are called genes, but other DNA sequences have structural purposes, or are involved in regulating the expression of genetic information.
DNA is a long polymer of simple units called nucleotides, which are held together by a backbone made of sugars and phosphate groups. This backbone carries four types of molecules called bases and it is the sequence of these four bases that encodes information. The major function of DNA is to encode the sequence of amino acid residues in proteins, using the genetic code. To read the genetic code, cells make a copy of a stretch of DNA in the nucleic acid RNA. These RNA copies can then be used to direct protein synthesis, but they can also be used directly as parts of ribosomes or spliceosomes. James D. Watson and Francis Crick produced the first accurate model of DNA structure in 1953 in their article The Molecular structure of Nucleic Acids. Watson and Crick proposed the central dogma of molecular biology in 1957, describing how proteins are produced from nucleic DNA. In 1962 Watson, Crick, and Maurice Wilkins jointly received the Nobel Prize.
Fritz Haber (1868-1934) was a German chemist, known as "the father of chemical warfare" due to his work in developing and deploying chlorine and other poisonous gases for use in World War I. Along with Carl Bosch, he developed the Haber process, which is the catalytic formation of ammonia from hydrogen and atmospheric nitrogen under conditions of high temperature and high pressure. This is considered an important milestone in industrial chemistry, and he received the 1918 Nobel Prize in Chemistry for this work. As part of his work in chemical warfare, he developed gas masks with absorbent filters, and formulated a mathematical relationship between gas concentration and necessary exposure time to induce death, known as Haber's rule. He is also associated with the development of the cyanide formulation, Zyklon B.
Gallium is a chemical element in the periodic table that has the symbol Ga and atomic number 31. A rare, soft silvery metallic poor metal, gallium is a brittle solid at low temperatures but liquefies slightly above room temperature and indeed will melt in the hand. It occurs in trace amounts in bauxite and zinc ores. An important application is in the compound gallium arsenide, used as a semiconductor, most notably in light-emitting diodes (LEDs).
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||