Arsenic
2008/9 Schools Wikipedia Selection. Related subjects: Chemical elements
|
|||||||||||||||||||||||||||||||||||||
| General | |||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
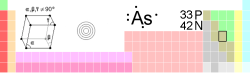
| Name, Symbol, Number | arsenic, As, 33 | ||||||||||||||||||||||||||||||||||||
| Chemical series | metalloids | ||||||||||||||||||||||||||||||||||||
| Group, Period, Block | 15, 4, p | ||||||||||||||||||||||||||||||||||||
| Appearance | metallic gray |
||||||||||||||||||||||||||||||||||||
| Standard atomic weight | 74.92160 (2) g·mol−1 | ||||||||||||||||||||||||||||||||||||
| Electron configuration | [Ar] 3d10 4s2 4p3 | ||||||||||||||||||||||||||||||||||||
| Electrons per shell | 2, 8, 18, 5 | ||||||||||||||||||||||||||||||||||||
| Physical properties | |||||||||||||||||||||||||||||||||||||
| Phase | solid | ||||||||||||||||||||||||||||||||||||
| Density (near r.t.) | 5.727 g·cm−3 | ||||||||||||||||||||||||||||||||||||
| Liquid density at m.p. | 5.22 g·cm−3 | ||||||||||||||||||||||||||||||||||||
| Melting point | 1090 K (817 ° C, 1503 ° F) |
||||||||||||||||||||||||||||||||||||
| Boiling point | subl. 887 K (614 ° C, 1137 ° F) |
||||||||||||||||||||||||||||||||||||
| Critical temperature | 1673 K | ||||||||||||||||||||||||||||||||||||
| Heat of fusion | (gray) 24.44 kJ·mol−1 | ||||||||||||||||||||||||||||||||||||
| Heat of vaporization | ? 34.76 kJ·mol−1 | ||||||||||||||||||||||||||||||||||||
| Specific heat capacity | (25 °C) 24.64 J·mol−1·K−1 | ||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||
| Atomic properties | |||||||||||||||||||||||||||||||||||||
| Crystal structure | rhombohedral | ||||||||||||||||||||||||||||||||||||
| Oxidation states | 5, 3, 1, -3 (mildly acidic oxide) |
||||||||||||||||||||||||||||||||||||
| Electronegativity | 2.18 (Pauling scale) | ||||||||||||||||||||||||||||||||||||
| Ionization energies ( more) |
1st: 947.0 kJ·mol−1 | ||||||||||||||||||||||||||||||||||||
| 2nd: 1798 kJ·mol−1 | |||||||||||||||||||||||||||||||||||||
| 3rd: 2735 kJ·mol−1 | |||||||||||||||||||||||||||||||||||||
| Atomic radius | 115 pm | ||||||||||||||||||||||||||||||||||||
| Atomic radius (calc.) | 114 pm | ||||||||||||||||||||||||||||||||||||
| Covalent radius | 119 pm | ||||||||||||||||||||||||||||||||||||
| Van der Waals radius | 185 pm | ||||||||||||||||||||||||||||||||||||
| Miscellaneous | |||||||||||||||||||||||||||||||||||||
| Magnetic ordering | no data | ||||||||||||||||||||||||||||||||||||
| Electrical resistivity | (20 °C) 333 n Ω·m | ||||||||||||||||||||||||||||||||||||
| Thermal conductivity | (300 K) 50.2 W·m−1·K−1 | ||||||||||||||||||||||||||||||||||||
| Young's modulus | 8 GPa | ||||||||||||||||||||||||||||||||||||
| Bulk modulus | 22 GPa | ||||||||||||||||||||||||||||||||||||
| Mohs hardness | 3.5 | ||||||||||||||||||||||||||||||||||||
| Brinell hardness | 1440 MPa | ||||||||||||||||||||||||||||||||||||
| CAS registry number | 7440-38-2 | ||||||||||||||||||||||||||||||||||||
| Selected isotopes | |||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||
| References | |||||||||||||||||||||||||||||||||||||
Arsenic (pronounced /ˈɑrsənɪk/) is a chemical element that has the symbol As and atomic number 33. Arsenic was first written about by Albertus Magnus (Germany) in 1250. Its Atomic Mass is 74.92. Its Ionic Charge is (3-) Its position in the periodic table is shown at right. This is a notoriously poisonous metalloid that has many allotropic forms: yellow (molecular non-metallic) and several black and gray forms (metalloids) are a few that are seen. Three metalloidal forms of arsenic with different crystal structures are found free in nature (the minerals arsenic sensu stricto and the much rarer arsenolamprite and pararsenolamprite), but it is more commonly found as arsenide and arsenate compounds. Several hundred such mineral species are known. Arsenic and its compounds are used as pesticides, herbicides, insecticides and various alloys.
The most common oxidation states for arsenic are -3 (arsenides: usually alloy-like intermetallic compounds), +3 (arsenates(III) or arsenites, and most organoarsenic compounds), and +5 (arsenates(V): the most stable inorganic arsenic oxycompounds). Arsenic also bonds readily to itself, forming, for instance, As-As pairs in the red sulfide realgar and square As43- ions in the arsenide skutterudite. In the +3 oxidation state, the stereochemistry of arsenic is affected by possession of a lone pair of electrons.
Notable characteristics
Arsenic is very similar chemically to its predecessor, phosphorus. Like phosphorus, it forms colourless, odourless, crystalline oxides As2O3 and As2O5 which are hygroscopic and readily soluble in water to form acidic solutions. Arsenic (V) acid, like phosphoric acid, is a weak acid. Like phosphorus, arsenic forms an unstable, gaseous hydride: arsine (AsH3). The similarity is so great that arsenic will partly substitute for phosphorus in biochemical reactions and is thus poisonous. However, in subtoxic doses, soluble arsenic compounds act as stimulants, and were once popular in small doses as medicinals by people in the mid 18th century.
When heated in air it oxidizes to arsenic trioxide; the fumes from this reaction have an odour resembling garlic. This odour can be detected on striking arsenide minerals such as arsenopyrite with a hammer. Arsenic (and some arsenic compounds) sublimes upon heating at atmospheric pressure, converting directly to a gaseous form without an intervening liquid state. The liquid state appears at 20 atmospheres and above, which explains why the melting point is higher than the boiling point . Elemental arsenic is found in many solid forms: the yellow form is soft, waxy and unstable, and is made of tetrahedral As4 molecules similar to the molecules of white phosphorus. The gray, black or 'metallic' forms have somewhat layered crystal structures with bonds extending throughout the crystal. They are brittle semiconductors with a metallic luster. The density of the yellow form is 1.97 g/cm³; rhombohedral 'gray arsenic' is much denser with a density of 5.73 g/cm³; the other metalloidal forms are similarly dense.
Applications
Lead hydrogen arsenate was used well into the 20th century as an insecticide on fruit trees. Its use sometimes resulted in brain damage to those working the sprayers. In the last half century, monosodium methyl arsenate (MSMA), a less toxic organic form of arsenic, has replaced lead arsenate's role in agriculture.
Scheele's Green, a copper arsenate, was used in the 19th century as a coloring agent in sweets.
The application of most concern to the general public is probably that of wood treated with chromated copper arsenate, also known as CCA or Tanalith. The vast majority of older pressure-treated wood was treated with CCA. CCA lumber is still in widespread use in many countries, and was heavily used during the latter half of the 20th century as a structural and outdoor building material. It was commonly used in situations where rot or insect infestation was a possibility. Although the use of CCA lumber was banned in many areas after studies showed that arsenic could leach out of the wood into the surrounding soil (from playground equipment, for instance), a risk is also presented by the burning of older CCA timber. The direct or indirect ingestion of wood ash from burnt CCA lumber has caused fatalities in animals and serious poisonings in humans; the lethal human dose is approximately 20 grams of ash. Scrap CCA lumber from construction and demolition sites may be inadvertently used in commercial and domestic fires. Protocols for safe disposal of CCA lumber do not exist evenly throughout the world; there is also concern in some quarters about the widespread landfill disposal of such timber.
During the 18th, 19th, and 20th centuries, a number of arsenic compounds have been used as medicines, including arsphenamine (by Paul Ehrlich) and arsenic trioxide (by Thomas Fowler). Arsphenamine as well as Neosalvarsan was indicated for syphilis and trypanosomiasis, but has been superseded by modern antibiotics. Arsenic trioxide has been used in a variety of ways over the past 200 years, but most commonly in the treatment of cancer. The US Food and Drug Administration in 2000 approved this compound for the treatment of patients with acute promyelocytic leukemia that is resistant to ATRA. It was also used as Fowler's solution in psoriasis.
Copper acetoarsenite was used as a green pigment known under many different names, including ' Paris Green' and 'Emerald Green'. It caused numerous arsenic poisonings.
Other uses;
- Various agricultural insecticides, termination and poisons.
- Used in animal feed, particularly in the US as a method of disease prevention and growth stimulation.
- Gallium arsenide is an important semiconductor material, used in integrated circuits. Circuits made using the compound are much faster (but also much more expensive) than those made in silicon. Unlike silicon it is direct bandgap, and so can be used in laser diodes and LEDs to directly convert electricity into light.
- Also used in bronzing and pyrotechny.
Occupational Exposures
Exposure to higher-than-average levels of arsenic can occur in some occupations placing workers at risk. Industries that use inorganic arsenic and its compounds include wood preservation, glass production, nonferrous metal alloys, and electronic semiconductor manufacturing. Inorganic arsenic is also found in coke oven emissions associated with the smelter industry.
History
The word arsenic is borrowed from the Persian word زرنيخ Zarnikh meaning "yellow orpiment". Zarnikh was borrowed by Greek as arsenikon, which means masculine or potent. Arsenic has been known and used in Persia and elsewhere since ancient times. As the symptoms of arsenic poisoning were somewhat ill-defined, it was frequently used for murder until the advent of the Marsh test, a sensitive chemical test for its presence. (Another less sensitive but more general test is the Reinsch test.) Due to its use by the ruling class to murder one another and its potency and discreetness, arsenic has been called the Poison of Kings and the King of Poisons.
During the Bronze Age, arsenic was often included in bronze, which made the alloy harder (so-called " arsenical bronze").
Albertus Magnus (Albert the Great, 1193-1280) is believed to have been the first to isolate the element in 1250. In 1649 Johann Schröder published two ways of preparing arsenic.
In the Victorian era, 'arsenic' (colourless, crystalline, soluble 'white arsenic') was mixed with vinegar and chalk and eaten by women to improve the complexion of their faces, making their skin paler to show they did not work in the fields. Arsenic was also rubbed into the faces and arms of women to 'improve their complexion'. The accidental use of arsenic in the adulteration of foodstuffs led to the Bradford sweet poisoning in 1858, which resulted in approximately 20 deaths and 200 people taken ill with arsenic poisoning.
Occurrence
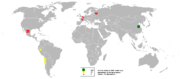
In 2005, China was the top producer of white arsenic with almost 50% world share followed by Chile and Peru, reports the British Geological Survey.
Arsenopyrite also unofficially called mispickel (FeAsS) is the most common arsenic-bearing mineral. On roasting in air, the arsenic sublimes as arsenic (III) oxide leaving iron oxides.
The most important compounds of arsenic are arsenic (III) oxide, As2O3, (' white arsenic'), the yellow sulfide orpiment (As2S3) and red realgar (As4S4), Paris Green, calcium arsenate, and lead hydrogen arsenate. The latter three have been used as agricultural insecticides and poisons. Orpiment and realgar were formerly used as painting pigments, though they have fallen out of use due to their toxicity and reactivity. Although arsenic is sometimes found native in nature, its main economic source is the mineral arsenopyrite mentioned above; it is also found in arsenides of metals such as silver, cobalt (cobaltite: CoAsS and skutterudite: CoAs3) and nickel, as sulfides, and when oxidised as arsenate minerals such as mimetite, Pb5(AsO4)3Cl and erythrite, Co3(AsO4)2. 8H2O, and more rarely arsenites ('arsenite' = arsenate(III), AsO33- as opposed to arsenate (V), AsO43-). In addition to the inorganic forms mentioned above, arsenic also occurs in various organic forms in the environment. Inorganic arsenic and its compounds, upon entering the food chain, are progressively metabolised to a less toxic form of arsenic through a process of methylation. For example certain molds produce significant amounts of trimethylarsine if inorganic arsenic is present.
Nickernuts are said to contain arsenic. See also Arsenide minerals, Arsenate minerals.
Toxicity
Arsenic and many of its compounds are especially potent poisons. Arsenic disrupts ATP production through several mechanisms. At the level of the citric acid cycle, arsenic inhibits pyruvate dehydrogenase and by competing with phosphate it uncouples oxidative phosphorylation, thus inhibiting energy-linked reduction of NAD+, mitochondrial respiration, and ATP synthesis. Hydrogen peroxide production is also increased, which might form reactive oxygen species and oxidative stress. These metabolic interferences lead to death from multi-system organ failure (see arsenic poisoning) probably from necrotic cell death, not apoptosis. A post mortem reveals brick red colored mucosa, due to severe hemorrhage. Although arsenic causes toxicity, it can also play a protective role..
Elemental arsenic and arsenic compounds are classified as " toxic" and "dangerous for the environment" in the European Union under directive 67/548/EEC.
The IARC recognizes arsenic and arsenic compounds as group 1 carcinogens, and the EU lists arsenic trioxide, arsenic pentoxide and arsenate salts as category 1 carcinogens.
Arsenic is known to cause arsenicosis due to its manifestation in drinking water, “the most common species being arsenate [HAsO42- ; As(V)] and arsenite [H3AsO3 ; As(III)]”. The ability of arsenic to undergo redox conversion between As(III) and As(V) makes its availability in the environment possible. According to Croal, Gralnick, Malasarn, and Newman, “[the] understanding [of] what stimulates As(III) oxidation and/or limits As(V) reduction is relevant for bioremediation of contaminated sites (Croal). The study of chemolithoautotrophic As(III) oxidizers and the heterotrophic As(V) reducers can help the understanding of the oxidation and/or reduction of arsenic.
Arsenic in drinking water
Arsenic contamination of groundwater has led to a massive epidemic of arsenic poisoning in Bangladesh and neighbouring countries. It is estimated that approximately 57 million people are drinking groundwater with arsenic concentrations elevated above the World Health Organization's standard of 10 parts per billion. The arsenic in the groundwater is of natural origin, and is released from the sediment into the groundwater due to the anoxic conditions of the subsurface. This groundwater began to be used after western NGOs instigated a massive tube well drinking-water program in the late twentieth century. This program was designed to prevent drinking of bacterially contaminated surface waters, but failed to test for arsenic in the groundwater.(2) Many other countries and districts in South East Asia, such as Vietnam, Cambodia, and Tibet, China, are thought to have geological environments similarly conducive to generation of high-arsenic groundwaters. Arsenicosis was reported in Nakhon Si Thammarat, Thailand in 1987, and the dissolved arsenic in the Chao Phraya River is suspected of containing high levels of naturally occurring arsenic, but has not been a public health problem due to the use of bottled water.
The northern United States, including parts of Michigan, Wisconsin, Minnesota and the Dakotas are known to have significant concentrations of arsenic in ground water. Increased levels of skin cancer has been associated with arsenic exposure in Wisconsin, even at levels below the 10 part per billion drinking water standard.
Epidemiological evidence from Chile shows a dose dependent connection between chronic arsenic exposure and various forms of cancer, particularly when other risk factors, such as cigarette smoking, are present. These effects have been demonstrated to persist below 50 parts per billion.
A study of cancer rates in Taiwan suggested that significant increases in cancer mortality appear only at levels above 150 parts per billion.
Analyzing multiple epidemiological studies on inorganic arsenic exposure suggests a small but measurable risk increase for bladder cancer at 10 parts per billion. According to Peter Ravenscroft, of the Department of Geography at the University of Cambridge roughly 80 million people worldwide consume between 10 and 50 parts per billion arsenic in their drinking water. If they all consumed exactly 10 parts per billion arsenic in their drinking water, the previously cited multiple epidemiological study analysis would predict an additional 2,000 cases of bladder cancer alone. This represents a clear underestimate of the overall impact, since it does not include lung or skin cancer, and explicitly underestimates the exposure. Those exposed to levels of arsenic above the current WHO standard should weigh the costs and benefits of arsenic remmediation.
Arsenic can be removed from drinking water through coprecipitation of iron minerals by oxidation and filtering. When this treatment fails to produce acceptable results, adsorptive arsenic removal media may be utilized. Several adsorptive media systems have been approved for point of service use in a study funded by the United States Environmental Protection Agency (U.S.EPA) and the National Science Foundation (NSF).
Magnetic separations of arsenic at very low magnetic field gradients have been demonstrated in point-of-use water purification with high–surface area and monodisperse magnetite (Fe3O4) nanocrystals. Using the high specific surface area of Fe3O4 nanocrystals the mass of waste associated with arsenic removal from water has been dramatically reduced.
Compounds
- Arsenic acid (H3AsO4)
- Arsenous acid (H3AsO3)
- Arsenic trioxide (As2O3)
- Arsine (Arsenic Trihydride AsH3)
- Cadmium arsenide (Cd3As2)
- Gallium arsenide (GaAs)
- Lead hydrogen arsenate (PbHAsO4)
Arsenic also occurs in the II oxidation state, but only in the As24+ cation, As(II) is never found otherwise.
Isotopes
Arsenic has been proposed as a " salting" material for nuclear weapons (cobalt is another, better-known salting material). A jacket of 75As, irradiated by the intense high-energy neutron flux from an exploding thermonuclear weapon, would transmute into the radioactive isotope 76As with a half-life of 1.0778 days and produce approximately 1.13 MeV of gamma radiation, significantly increasing the radioactivity of the weapon's fallout for several hours. Such a weapon is not known to have ever been built, tested, or used.