Cobalt
2008/9 Schools Wikipedia Selection. Related subjects: Chemical elements
|
|||||||||||||||||||||||||||||||||||||
| General | |||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Name, symbol, number | cobalt, Co, 27 | ||||||||||||||||||||||||||||||||||||
| Chemical series | transition metals | ||||||||||||||||||||||||||||||||||||
| Group, period, block | 9, 4, d | ||||||||||||||||||||||||||||||||||||
| Appearance | metallic with gray tinge |
||||||||||||||||||||||||||||||||||||
| Standard atomic weight | 58.933195 (5) g·mol−1 | ||||||||||||||||||||||||||||||||||||
| Electron configuration | [Ar] 4s2 3d7 | ||||||||||||||||||||||||||||||||||||
| Electrons per shell | 2, 8, 15, 2 | ||||||||||||||||||||||||||||||||||||
| Colour | a grayish silver | ||||||||||||||||||||||||||||||||||||
| Density (near r.t.) | 8.90 g·cm−3 | ||||||||||||||||||||||||||||||||||||
| Liquid density at m.p. | 7.75 g·cm−3 | ||||||||||||||||||||||||||||||||||||
| Melting point | 1768 K (1495 ° C, 2723 ° F) |
||||||||||||||||||||||||||||||||||||
| Boiling point | 3200 K (2927 ° C, 5301 ° F) |
||||||||||||||||||||||||||||||||||||
| Heat of fusion | 16.06 kJ·mol−1 | ||||||||||||||||||||||||||||||||||||
| Heat of vaporization | 377 kJ·mol−1 | ||||||||||||||||||||||||||||||||||||
| Specific heat capacity | (25 °C) 24.81 J·mol−1·K−1 | ||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||
| Atomic properties | |||||||||||||||||||||||||||||||||||||
| Crystal structure | hexagonal | ||||||||||||||||||||||||||||||||||||
| Oxidation states | 4 , 3, 2, 1 ( amphoteric oxide) |
||||||||||||||||||||||||||||||||||||
| Electronegativity | 1.88 (Pauling scale) | ||||||||||||||||||||||||||||||||||||
| Ionization energies ( more) |
1st: 760.4 kJ·mol−1 | ||||||||||||||||||||||||||||||||||||
| 2nd: 1648 kJ·mol−1 | |||||||||||||||||||||||||||||||||||||
| 3rd: 3232 kJ·mol−1 | |||||||||||||||||||||||||||||||||||||
| Atomic radius | 135 pm | ||||||||||||||||||||||||||||||||||||
| Atomic radius (calc.) | 152 pm | ||||||||||||||||||||||||||||||||||||
| Covalent radius | 126 pm | ||||||||||||||||||||||||||||||||||||
| Miscellaneous | |||||||||||||||||||||||||||||||||||||
| Magnetic ordering | ferromagnetic | ||||||||||||||||||||||||||||||||||||
| Electrical resistivity | (20 °C) 62.4 nΩ·m | ||||||||||||||||||||||||||||||||||||
| Thermal conductivity | (300 K) 100 W·m−1·K−1 | ||||||||||||||||||||||||||||||||||||
| Thermal expansion | (25 °C) 13.0 µm·m−1·K−1 | ||||||||||||||||||||||||||||||||||||
| Speed of sound (thin rod) | (20 °C) 4720 m/s | ||||||||||||||||||||||||||||||||||||
| Young's modulus | 209 GPa | ||||||||||||||||||||||||||||||||||||
| Shear modulus | 75 GPa | ||||||||||||||||||||||||||||||||||||
| Bulk modulus | 180 GPa | ||||||||||||||||||||||||||||||||||||
| Poisson ratio | 0.31 | ||||||||||||||||||||||||||||||||||||
| Mohs hardness | 5.0 | ||||||||||||||||||||||||||||||||||||
| Vickers hardness | 1043 MPa | ||||||||||||||||||||||||||||||||||||
| Brinell hardness | 700 MPa | ||||||||||||||||||||||||||||||||||||
| CAS registry number | 7440-48-4 | ||||||||||||||||||||||||||||||||||||
| Selected isotopes | |||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||
| References | |||||||||||||||||||||||||||||||||||||
Cobalt (pronounced /ˈkoʊbɒlt/) is a hard, lustrous, silver-grey metal, a chemical element with symbol Co. It is found in various ores, and is used in the preparation of magnetic, wear-resistant, and high-strength alloys. Its compounds are used in the production of inks, paints, and varnishes.
Notable characteristics
Cobalt is a silver or gray ferromagnetic metal. Pure cobalt is not found in nature, but compounds of cobalt occur naturally in many forms. Small amounts of it are found in most rocks, soil, water, plants, and animals. It is an element of atomic number 27. The Curie temperature is of 1388 K with 1.6~1.7 Bohr magnetons per atom. In nature, it is frequently associated with nickel, and both are characteristic ingredients of meteoric iron. Mammals require small amounts of cobalt which is the basis of vitamin B12. Cobalt-60, an artificially produced radioactive isotope of cobalt, is an important radioactive tracer and cancer-treatment agent. Cobalt has a relative permeability two thirds that of iron. Metallic cobalt commonly presents a mixture of two crystallographic structures hcp and fcc with a transition temperature hcp→fcc of 722 K. Cobalt has a hardness of 5.5 on the Mohs scale of mineral hardness.
Common oxidation states of cobalt include +2 and +3, although compounds with oxidation state +1 are also well developed.
Isotopes
Naturally occurring cobalt is "monoisotopic"; i.e. only one isotope is stable: 59Co. 22 radioisotopes have been characterized with the most stable being 60Co with a half-life of 5.2714 years, 57Co with a half-life of 271.79 days, 56Co with a half-life of 77.27 days, and 58Co with a half-life of 70.86 days. All of the remaining radioactive isotopes have half-lives that are less than 18 hours and the majority of these have half-lives that are less than 1 second. This element also has 4 meta states, all of which have half-lives less than 15 minutes.
The isotopes of cobalt range in atomic weight from 50 u (50Co) to 73 u (73Co). The primary decay mode for isotopes with atomic mass unit values less than that of the most abundant stable isotope, 59Co, is electron capture and the primary mode of decay for those of greater than 59 atomic mass units is beta decay. The primary decay products before 59Co are element 26 (iron) isotopes and the primary products after are element 28 (nickel) isotopes.
Cobalt radioisotopes in medicine
Cobalt-60 (Co-60 or 60Co) is a radioactive metal that is used in radiotherapy. It produces two gamma rays with energies of 1.17 MeV and 1.33 MeV. The 60Co source is about 2 cm in diameter and as a result produces a geometric penumbra, making the edge of the radiation field fuzzy. The metal has the unfortunate habit of producing a fine dust, causing problems with radiation protection. The 60Co source is useful for about 5 years but even after this point is still very radioactive, and so cobalt machines have fallen from favour in the Western world where linacs are common.
Cobalt-57 (Co-57 or 57Co) is a radioactive metal that is used in medical tests; it is used as a radiolabel for vitamin B12 uptake. It is useful for the Schilling test.
Industrial uses for radioactive isotopes
Cobalt-60 (Co-60 or 60Co) is useful as a gamma ray source because it can be produced—in predictable quantity, and high activity—by simply exposing natural cobalt to neutrons in a reactor for a given time. It is used for
- sterilization of medical supplies, and medical waste;
- radiation treatment of foods for sterilization (cold pasteurization);
- industrial radiography (e.g., weld integrity radiographs);
- density measurements (e.g., concrete density measurements); and
- tank fill height switches.
Cobalt-59 is used as a source in Mössbauer spectroscopy.
Applications
- Alloys, such as
- Magnets and magnetic recording media.
- Alnico magnets.
- Samarium-cobalt magnets.
- Catalysts for the petroleum and chemical industries, e.g. for hydroformylation and oxidation.
- Electroplating because of its appearance, hardness, and resistance to oxidation.
- Drying agents for paints, varnishes, and inks.
- Ground coats for porcelain enamels.
- Pigments ( cobalt blue and cobalt green).
- Lithium ion battery electrodes.
- Steel-belted radial tires.
- Purification of histidine-tagged fusion proteins in biotechnology applications.
History
Cobalt compounds have been used for centuries to impart a rich blue colour to glass, glazes, and ceramics. Cobalt has been detected in Egyptian sculpture and Persian jewelry from the third millennium BC, in the ruins of Pompeii (destroyed AD 79), and in China dating from the Tang dynasty (AD 618–907) and the Ming dynasty (AD 1368–1644). Cobalt glass ingots have been recovered from shipwrecks dating to the time of the Minoans (BC 2700-1450).
Swedish chemist George Brandt (1694–1768) is credited with isolating cobalt in 1735. He was able to show that cobalt was the source of the blue colour in glass, which previously had been attributed to the bismuth found with cobalt.
During the 19th century, cobalt blue was produced at the Norwegian Blaafarveværket (70-80% of world production), led by the Prussian industrialist Benjamin Wegner.
In 1938, John Livingood and Glenn Seaborg discovered cobalt-60.
The word cobalt is derived from the German kobalt, from kobold meaning "goblin", a term used for the ore of cobalt by miners. The first attempts at smelting the cobalt ores to produce cobalt metal failed, yielding cobalt(II) oxide instead; not only that, but because of cobalt's curious affinity for arsenic, the primary ores of cobalt always contain arsenic, and upon smelting the arsenic oxidized into the highly toxic As4O6, which was breathed in by workers.
Occurrence
Cobalt is not found as a native metal but generally found in the form of ores. Cobalt is usually not mined alone, and tends to be produced as a by-product of nickel and copper mining activities. The main ores of cobalt are cobaltite, erythrite, glaucodot, and skutterudite.
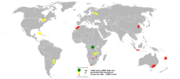
In 2005, the Democratic Republic of the Congo was the top producer of cobalt with almost 40% world share followed by Canada, Zambia, Russia, Brazil and Cuba, reports the British Geological Survey.
Compounds
There is a wide variety of cobalt compounds. The +2 and +3 oxidation states are most prevalent, however cobalt(I) complexes are also fairly common. Cobalt(II) salts form the red-pink [Co(OH2)6]2+ complex in aqueous solution. Adding excess chloride will also change the colour from pink to blue, due to the formation of [CoCl4]2-. Cobalt oxides are antiferromagnetic at low temperature: CoO ( Neel temperature 291 K) and Co3O4 (Neel temperature: 40 K), which is analogous to magnetite (Fe3O4), with a mixture of +2 and +3 oxidation states. The oxide Co2O3 is probably unstable; it has never been synthesized. Other than Co3O4 and the brown fluoride CoF3 (which is instantly hydrolyzed in water), all compounds containing cobalt in the +3 oxidation state are stabilized by complex ion formation.
- see also Category:Cobalt compounds
Biological role
Cobalt in small amounts is essential to many living organisms, including humans. Having 0.13 to 0.30 mg/kg of cobalt in soils markedly improves the health of grazing animals. Cobalt is a central component of the vitamin cobalamin, or vitamin B12.
Isotopes
60Co is a high-energy gamma ray emitter. Acute high-dose exposures to the gamma emissions can cause severe burns and death. Extended exposures increase the risk of morbidity or mortality from cancer.
Nuclear weapon designs could intentionally incorporate 59Co, some of which would be activated in a nuclear explosion to produce 60Co. The 60Co, dispersed as nuclear fallout, creates what is sometimes called a dirty bomb or cobalt bomb.
Precautions
Although cobalt is an essential element for life in minute amounts, at higher levels of exposure it shows mutagenic and carcinogenic effects similar to nickel (see Cobalt Poisoning ).
Powdered cobalt in metal form is a fire hazard.