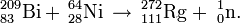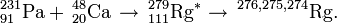Roentgenium
2008/9 Schools Wikipedia Selection. Related subjects: Chemical elements
|
|||||||||||||||||||||||||||||||||||||
| General | |||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Name, Symbol, Number | roentgenium, Rg, 111 | ||||||||||||||||||||||||||||||||||||
| Element category | transition metals | ||||||||||||||||||||||||||||||||||||
| Group, Period, Block | 11, 7, d | ||||||||||||||||||||||||||||||||||||
| Appearance | unknown, predicted colourless | ||||||||||||||||||||||||||||||||||||
| Standard atomic weight | [280] g·mol−1 | ||||||||||||||||||||||||||||||||||||
| Electron configuration | predicted [Rn] 5f14 6d9 7s2 | ||||||||||||||||||||||||||||||||||||
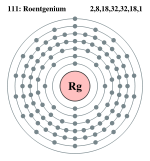
| Electrons per shell | 2, 8, 18, 32, 32, 17, 2 | ||||||||||||||||||||||||||||||||||||
| Phase | presumably a solid | ||||||||||||||||||||||||||||||||||||
| CAS registry number | 54386-24-2 | ||||||||||||||||||||||||||||||||||||
| Selected isotopes | |||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||
| References | |||||||||||||||||||||||||||||||||||||
Roentgenium (pronounced /rɛntˈgɛniəm/, /rʌntˈdʒɛniəm/) is a chemical element in the periodic table that has the symbol Rg and atomic number 111.
It is a synthetic element whose most stable known isotope has a mass of 283 and a half-life of ten minutes.
Official discovery
Element 111 was officially discovered by Peter Armbruster, Gottfried Münzenber, and their team working at the Gesellschaft für Schwerionenforschung (GSI) in Darmstadt, Germany on December 8, 1994. Only three atoms of it were observed (all 272Rg), by the cold fusion between 64Ni ions and a 209Bi target in a linear accelerator:
In 2001, the IUPAC/IUPAP Joint Working Party (JWP) from concluded that there was insufficient evidence for the discovery at that moment in time. The GSI team repeated their experiment in 2000 and detected a further 3 atoms. In their 2003 report, the JWP decided that the GSI team should be acknowledged as the discoverers.
Proposed name
The name roentgenium (Rg) was proposed by the GSI team and was accepted as a permanent name on November 1, 2004 in honour of the German physicist Wilhelm Conrad Röntgen. Previously the element was known under the temporary IUPAC systematic element name unununium (pronounced /ˌjuːnənˈjuːniəm/ or /ˌʌnəˈnʌniəm/), Uuu. Some research has referred to it as eka-gold.
The official baptism took place at GSI, on Friday November 17, 2006, in presence of Annette Schavan, the Federal German Minister of Research.
Electronic structure
Non-relativistic
Roentgenium has 6 full shells, 7 s+5 p+3 d+2 f=17 full subshells, and 111 orbitals:
Bohr model: 2, 8, 18, 32, 32, 18, 1
Quantum mechanical model: 1s22s22p63s23p64s23d10 4p65s24d105p66s24f145d10 6p67s15f146d10
Relativistic
The stable group 11 elements, copper, silver, and gold all have an outer electron configuration nd10(n+1)s1. For each of these elements, their first excited state has a configuration nd9(n+1)s2. Due to spin-orbit coupling between the s electrons, this state is split into a pair of energy levels. For copper, the difference in energy between the ground state and lowest excited state causes the metal to appear reddish. For silver, the energy gap widens and it become silvery. However, as Z increases, the excited levels are stabilised by relativistic effects and in gold the energy gap decreases again and it appears gold. For roentgenium, calculations indicate that the 6d97s2 level is stabilised to such an extent that it becomes the ground state. The resulting energy difference between the new ground state and the first excited state is similar to that of silver and roentgenium is expected to be silvery in appearance.
Extrapolated chemical properties of eka-gold/dvi-silver
Oxidation states
Element 111 is projected to be the ninth member of the 6d series of transition metals and the heaviest member of group 11 (IB) in the Periodic Table, below copper, silver, and gold. Each of the members of this group show different stable states. Copper forms a stable +II state, whilst silver is predominantly found as Ag(I) and gold as Au(III). Copper(I) and silver(II) are also relatively well-known. Roentgenium is therefore expected to predominantly form a stable +III state.
Chemistry
The heavier members of this group are well known for their lack of reactivity or noble character. Silver and gold are both inert to oxygen. They are both however attacked by the halogens. In addition, silver is attacked by sulfur and hydrogen sulfide, highlighting its higher reactivity to gold. Roentgenium is expected to be even more noble than gold and can be expected to be inert to oxygen and halogens. The most-likely reaction is with fluorine to form a trifluoride, RgF3.
History of synthesis of isotopes by cold fusion
209Bi(64Ni,xn)273−xRg (x=1)
First experiments to synthesize element 111 were performed by the Dubna team in 1986 using this cold fusion reaction. No atoms were identified that could be assigned to atoms of element 111 and a production cross-section limit of 4 pb was determined. After an upgrade of their facilities, the team at GSI successfully detected 3 atoms of 272Rg in their discovery experiment. A further 3 atoms were synthesized in 2000. The discovery of roentgenium was confirmed in 2003 when a team at RIKEN measured the decays of 14 atoms of 272Rg during the measurement of the 1n excitation function.
208Pb(65Cu,xn)273−xRg (x=1)
In 2004, as part of their study of odd-Z projectiles in cold fusion reactions, the team at LBNL detected a single atom of 272Rg in this new reaction.
History of synthesis of isotopes as decay products
Isotopes of roentgenium have also been observed in the decay of heavier elements. Observations to date are outlined in the table below:
| Evaporation residue | Observed Rg isotope |
|---|---|
| 288115 | 280Rg |
| 287115 | 279Rg |
| 282113 | 278Rg |
| 278113 | 274Rg |
Chronology of isotope discovery
| Isotope | Year discovered | Discoverer reaction |
|---|---|---|
| 272Rg | 1994 | 209Bi(64Ni,n) |
| 273Rg | unknown | |
| 274Rg | 2004 | 209Bi(70Zn,n) |
| 275Rg | unknown | |
| 276Rg | unknown | |
| 277Rg | unknown | |
| 278Rg | 2006 | 237Np(48Ca,3n) |
| 279Rg | 2003 | 243Am(48Ca,4n) |
| 280Rg | 2003 | 243Am(48Ca,3n) |
Chemical yields of isotopes
Cold fusion
The table below provides cross-sections and excitation energies for cold fusion reactions producing roentgenium isotopes directly. Data in bold represents maxima derived from excitation function measurements. + represents an observed exit channel.
| Projectile | Target | CN | 1n | 2n | 3n |
|---|---|---|---|---|---|
| 64Ni | 209Bi | 3.5 pb, 12.5 MeV | |||
| 65Cu | 208Pb | 273Rg | 1.7 pb, 13.2 MeV |
Isotopes
Five isotopes of roentgenium are known. The longest-lived of these is 280Rg, which decays through alpha decay and has a halflife of 3.6 seconds. The shortest-lived isotope is 272Rg, which decays through alpha decay and has a halflife of 1.6 ms.
Isomerism in roentgenium nuclides
274Rg
Two atoms of 274Rg have been observed in the decay chains starting with 278113. The two events occur with different energies and with different lifetimes. In addition, the two entire decay chains appear to be different. This suggests the presence of two isomeric levels but further research is required.
272Rg
The direct production of 272Rg has provided four alpha lines at 11.37, 11.03, 10.82, and 10.40 MeV. The GSI measured a half-life of 1.6 ms whilst recent data from RIKEN has given a half-life of 3.8 ms. The conflicting data may be due to isomeric levels but the current data are insufficient to come to any firm assignments.
Future experiments
To date there has been no attempt to synthesise roentgenium in hot fusion reactions. It has been mentioned by the Dubna team that they could complete their Ca-48 projectile program by studying the reaction