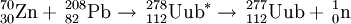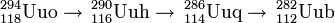Ununbium
2008/9 Schools Wikipedia Selection. Related subjects: Chemical elements
|
||||||||||||||||||||||||||||||||||||||||
| General | ||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Name, Symbol, Number | ununbium, Uub, 112 | |||||||||||||||||||||||||||||||||||||||
| Chemical series | transition metals | |||||||||||||||||||||||||||||||||||||||
| Group, Period, Block | 12, 7, d | |||||||||||||||||||||||||||||||||||||||
| Appearance | unknown, probably silvery white or metallic gray liquid or colorless gas |
|||||||||||||||||||||||||||||||||||||||
| Standard atomic weight | [285] g·mol−1 | |||||||||||||||||||||||||||||||||||||||
| Electron configuration | [Rn] 5f14 6d10 7s2 |
|||||||||||||||||||||||||||||||||||||||
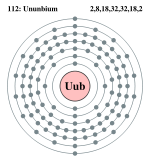
| Electrons per shell | 2, 8, 18, 32, 32, 18, 2 | |||||||||||||||||||||||||||||||||||||||
| Phase | unknown | |||||||||||||||||||||||||||||||||||||||
| CAS registry number | 54084-26-3 | |||||||||||||||||||||||||||||||||||||||
| Selected isotopes | ||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
| References | ||||||||||||||||||||||||||||||||||||||||
Ununbium (pronounced /juːˈnʌnbiəm/ or /əˈnʌnbiəm/) is a temporary IUPAC systematic element name for a chemical element in the periodic table that has the temporary symbol Uub and the atomic number 112.
Element 112 is one of the superheavy elements. Recent experiments strongly suggest that element 112 behaves as a typical member of group 12, demonstrating properties consistent with a volatile metal.
Discovery Profile
Claims of synthesizing this element have been made in 1971.
Ununbium was reportedly first created on February 9, 1996 at the Gesellschaft für Schwerionenforschung (GSI) in Darmstadt, Germany by Sigurd Hofmann, Victor Ninov et al. This element was created by firing accelerated zinc-70 nuclei at a target made of lead-208 nuclei in a heavy ion accelerator. A single atom (the second has subsequently been dismissed) of ununbium was produced with a mass number of 277.
In May 2000, the GSI successfully repeated the experiment to synthesise a further atom of Uub-277. This reaction was repeated at RIKEN using the GARIS set-up in 2004 to synthesise two further atoms and confirm the decay data reported by the GSI team. In a quantum tuneling model the alpha-decay chains from 277112 have been studied and the calculated results furnish corroborating evidence for the experimental findings at RIKEN and GSI.
The IUPAC/IUPAP Joint Working Party (JWP) assessed the claim of discovery by the GSI team in 2001 and 2003. In both cases, they found that there was insufficient evidence to support their claim. This was primarily related to the contradicting decay data for the known isotope 261Rf. However, between 2001-2005, the GSI team studied the reaction 248Cm(26Mg,5n)269Hs, and were able to confirm the decay data for 269Hs and 261Rf. It was found that the existing data on 261Rf was for a meta-stable isomer, namely 261mRf.
The JWP has completed the re-assessment of the claim of discovery by the various teams and has written a draft report which should be published as a technical report in Pure and Applied Chemistry in early 2008.
Naming
Current names
The element with Z=112 is historically known as eka-mercury. Ununbium (Uub) is a temporary IUPAC systematic element name. Research scientists usually refer to the element simply as element 112 (E112).
Proposed names by claimants
Claims to the discovery of element 112 have been put forward by Dmitriev of the Dubna team, Morita of the RIKEN team and Hofmann of the GSI team. The JWP will decide to whom the right to suggest a name will be given. The IUPAC have the final say on the official adoption of a name. No name has been suggested as yet by any of the claimant laboratories.
Disallowed names
According to IUPAC rules, names used for previous elements that have ultimately not been adopted are not allowed to be proposed for future use. The table below summarises those names which are probably not allowed to be proposed by the claimant laboratories under the rules.
| Group Claim Affected | Disallowed Name | Disallowed Symbol | Reason |
|---|---|---|---|
| RIKEN | Nipponium | Np | Used for claimed discovery of element 43 |
| Dubna | Russium | Rs | Used for claimed discovery of element 43 |
| GSI | Hahnium | Ha , Hn | Used for claimed discovery of element 105 and 108 |
Plausible names
Many speculative names appear in popular literature. The table below lists these names in the case where they obey IUPAC rules and are plausible with regard to the claimant laboratories. Rumoured suggestions (*) linked to the claimant laboratories are also included.
| Group Link | Name | Symbol | Derivation |
|---|---|---|---|
| GSI | Wixhausium * | Wi | Wixhausen - Suburb of Darmstadt |
| GSI | Helmholtzium * | Hh | Hermann von Helmholtz - prominent German chemist |
| GSI | Venusium | Vs | Venus - reference to eka-mercury |
| GSI | Frischium | Fs | Otto Frisch - Austrian physicist co-founder of fission with Lise Meitner |
| GSI | Strassmanium | St | Fritz Strassman - German physicist co-discoverer of fission with Otto Hahn |
| GSI | Heisenbergium | Hb | Werner Heisenberg - German nuclear physicist |
Electronic structure
Ununbium is element 112 in the Periodic Table. The two forms of the projected electronic structure are:
Bohr model: 2, 8, 18, 32, 32, 18, 2
Quantum mechanical model: 1s22s22p63s23p64s23d10 4p65s24d105p66s24f145d10 6p67s25f146d10
Extrapolated chemical properties of eka-mercury/dvi-cadmium
Oxidation states
Element 112 is projected to be the last member of the 6d series of transition metals and the heaviest member of group 12 (IIB) in the Periodic Table, below zinc, cadmium and mercury. Each of the members of this group show a stable +II oxidation state. In addition, mercury(I) is also well known. Element 112 is therefore expected to form a stable +II state.
Chemistry
The known members of group 12 all react with oxygen and sulfur directly to form the oxides and sulfides, MO and MS, respectively. Mercury(II) oxide, HgO, can be decomposed by heat to the liquid metal. Mercury also has a well known affinity for sulfur. Therefore, element 112 should form an analogous oxide [112]O and sulfide [112]S. In their halogen chemistry, all the metals form the ionic difluoride MF2 upon reaction with fluorine. The other halides are known but for mercury, the soft nature of the Hg(II) ion leads to a high degree of covalency and HgCl2, HgBr2 and HgCl2 are low-melting, volatile solids. Therefore, element 112 is expected to form an ionic fluoride, [112]F2, but volatile halides, [112]Cl2, [112]Br2 and [112]I2. In addition, mercury is well known for its alloying properties, with the concomitant formation of amalgams, especially with gold and silver. It is also a volatile metal and is monatomic in the vapour phase. Element 112 is therefore also predicted to be a volatile metal which readily combines with gold to form a Au-E112 metal-metal bond.
Experimental chemistry
Atomic gas phase
Ununbium is expected to have the ground state electron configuration [Rn]5f14 6d10 7s2 and thus belong to group 12 of the Periodic Table. As such, it should behave as the heavier homologue of mercury (Hg) and form strong binary compounds with noble metals like gold. Experiments probing the reactivity of ununbium have focused on the adsorption of atoms of element 112 onto a gold surface held at varying temperatures, in order to calculate an adsorption enthalpy. Due to possible relativistic stabilisation of the 7s electrons, leading to radon-like properties, experiments were performed with the simultaneous formation of mercury and radon radioisotopes, allowing a comparison of adsorption characteristics.
The first experiments were conducted using the 238U(48Ca,3n)283112 reaction. Detection was by spontaneous fission of the claimed 5 min parent isotope. Analysis of the data indicated that ununbium was more volatile than mercury and had noble-gas properties. However, the confusion regarding the synthesis of 283112 has cast some doubt on these experimental results.
Given this uncertainty, between April-May 2006 at the JINR, a FLNR-PSI team conducted experiments probing the synthesis of this isotope as a daughter in the nuclear reaction 242Pu(48Ca,3n)287114. In this experiment, two atoms of 283112 were unambiguously identified and the adsorption properties indicated that ununbium is a more volatile homologue of mercury, due to formation of a weak metal-metal bond with gold, placing it firmly in group 12.
In April 2007 this experiment was repeated and a further 3 atoms of 283112 were positively identified. The adsorption property was confirmed and indicated that element 112 has adsorption properties completely in agreement with being the heaviest member of group 12.
History of synthesis of isotopes by cold fusion
208Pb(70Zn,xn)278-x112 (x=1)
The team at GSI first studied this reaction in 1996 and detected two decay chains of 277112. In a review of the data in 2000, the first decay chain was retracted. In a repeat of the reaction in 2000 they were able to synthesise a further atom. They attempted to measure the 1n excitation function in 2002 but suffered from a failure of the Zn-70 beam. The unofficial discovery of 277112 was confirmed in 2004 at RIKEN who detected a further 2 atoms of the isotope and were able to confirm the decay data for the entire chain.
208Pb(68Zn,xn)276-x112
Following the successful synthesis of 277Uub, the GSI team performed a reaction using a 68Zn projectile in 1997 in an effort to study the effect of isospin (neutron richness) on the chemical yield. The experiment was initiated following the discovery of a yield enhancement during the synthesis of darmstadtium isotopes using 62Ni and 64Ni ions. No decay chains of 275112 were detected leading to a cross section limit of 1.2 pb. However, the revision of the yield for the 70Zn reaction to 0.5 pb does not rule out a similar yield for this reaction.
184W(88Sr,xn)272-x112
In 1990, after some early indications for the formation of isotopes of element 112 in the irradiation of a tungsten target with multi-GeV protons, a collaboration between GSI and the University of Jerusalem studied the above reaction. They were able to detect some spontaneous fission activity and a 12.5 MeV alpha decay, both of which they tentatively assigned to the radiative capture product 272112 or the 1n evaporation residue 271112. Both the TWG and JWP have concluded that a lot more research is required to confirm these conclusions.
History of synthesis of isotopes by hot fusion
238U(48Ca,xn)286-x112 (x=3,4)
In 1998, the team at the Flerov Laboratory of Nuclear Research began a research program using Ca-48 nuclei in "warm" fusion reactions leading to superheavy elements (SHE's). In March 1998, they claimed to have synthesised the element (2 atoms) in this reaction. The product, 283Uub, had a claimed half-life of 5 min, decaying by spontaneous fission (SF).
The long lifetime of the product initiated first chemical experiments on the gas phase atomic chemistry of element 112. In 2000, Yuri Yukashev at Dubna repeated the experiment but was unable to observe any spontaneous fission from 5 min activities. The experiment was repeated in 2001 and an accumulation of 8 SF fragments were found in the low temperature section, indicating that ununbium had radon-like properties. However, there is now some serious doubt about the origin of these results.
In order to confirm the synthesis, the reaction was successfully repeated by the same team in Jan 2003, confirming the decay mode and half life. They were also able to calculate an estimate of the mass of the SF activity to ~285 lending support to the assignment.
The team at LBNL entered the debate and performed the reaction in 2002. They were unable to detect any SF activities and calculated a cross section limit of 1.6 pb for the detection of a single event.
The reaction was repeated in 2003-2004 by the team at Dubna using a slightly different set-up, the Dubna Gas Filled Recoil Separator (DGFRS). This time, 283Uub was found to decay by emission of a 9.53 MeV alpha-particle with a half-life of 4 seconds. 282Uub was also observed in the 4n channel.
In 2003, the team at GSI entered the debate and performed a search for the 5 minute SF activity in chemical experiments. Like the Dubna team, they were able to detect 7 SF fragments in the low temperature section. However, these SF events were uncorrelated, suggesting they were not from actual direct SF of element 112 nuclei and raised doubts about the original indications for radon-like properties. After the announcement from Dubna of different decay properties for 283112, the GSI team repeated the experiment in September 2004. They were unable to detect any SF events and calculated a cross section limit of ~ 1.6 pb for the detection of one event, not in contradiction with the reported 2.5 pb yield by Dubna.
In May 2005, the GSI performed a physical experiment and identified a single atom of 283112 decaying by SF with a short lifetime suggesting a previously unknown SF branch. However, initial work by Dubna had detected several direct SF events but had assumed that the parent alpha decay had been missed. These results indicated that this was not the case.
In 2006, the new decay data on 283112 was confirmed by a joint PSI-FLNR experiment aimed at probing the chemical properties of ununbium. Two atoms of 283Uub were observed in the decay of the parent 287Uuq nuclei. The experiment indicated that contrary to previous experiments, element 112 behaves as a typical member of group 12, demonstrating properties of a volatile metal.
Finally, the team at GSI successfully repeated their physical experiment in Jan 2007 and detected 3 atoms of 283112, confirming both the alpha and SF decay modes.S. Hofmann et al. (2007). "The reaction 48Ca + 238U -> 286112* studied at the GSI-SHIP". Eur. Phys. J. A 32 (3): 251-260. doi:.</ref>
As such, the 5 min SF activity is still unconfirmed and unidentified. It is possible that it refers to a meta-stable isomer, namely 283mUub, whose yield is obviously dependent upon the exact production methods.
233U(48Ca,xn)281-x112
The team at FLNR studied this reaction in 2004. They were unable to detect any atoms of element 112 and calculated a cross section limit of 600 fb. The team concluded that this indicated that the neutron mass number for the compound nucleus had an effect on the yield of evaporation residues.
Synthesis of isotopes as decay products
Element 112 has also been observed as decay products of elements 114, 116 and 118 (see ununoctium).
| Evaporation Residue | Observed Uub isotope |
|---|---|
| 293116 , 289114 | 285112 |
| 292116 , 288114 | 284112 |
| 291116 , 287114 | 283112 |
| 294118 , 290116 , 286114 | 282112 |
As an example, in May 2006, the Dubna team ( JINR) identified 282Uub as a final product in the decay of ununoctium via the alpha decay sequence:
It was found that the final nucleus undergoes spontaneous fission.
Chronology of isotope discovery
| Isotope | Year discovered | discovery reaction |
|---|---|---|
| 277Uub | 1996 | 208Pb(70Zn,n) |
| 278Uub | unknown | |
| 279Uub | unknown | |
| 280Uub | unknown | |
| 281Uub | unknown | |
| 282Uub | 2004 | 238U(48Ca,4n) |
| 283Uub | 2002 | 244Pu(48Ca,5n) |
| 284Uub | 2002 | 244Pu(48Ca,4n) |
| 285Uub | 1999 | 244Pu(48Ca,3n) |
Chemical yields of isotopes
Cold fusion
The table below provides cross-sections and excitation energies for cold fusion reactions producing ununbium isotopes directly. Data in bold represents maxima derived from excitation function measurements. + represents an observed exit channel.
| Projectile | Target | CN | 1n | 2n | 3n |
|---|---|---|---|---|---|
| 70Zn | 208Pb | 278Uub | 0.5 pb , 10.0;12.0 MeV | ||
| 68Zn | 208Pb | 276Uub | < 1.2 pb , 11.3;12.8 MeV |
Hot fusion
The table below provides cross-sections and excitation energies for hot fusion reactions producing ununbium isotopes directly. Data in bold represents maxima derived from excitation function measurements. + represents an observed exit channel.
| Projectile | Target | CN | 3n | 4n | 5n |
|---|---|---|---|---|---|
| 48Ca | 238U | 286Uub | 2.5 pb , 35.0 MeV | 0.6 pb | |
| 48Ca | 233U | 281Uub | < 0.6 pb , 34.9 MeV |
Isomerism in ununbium nuclides
285112
In the synthesis of 289114 and 293116, a 9.15 MeV alpha-decaying activity has been detected with a half-life of 8.9 minutes. Although unconfirmed in recent experiments, it is highly possible that this is associated with a meta-stable isomer, namely 285m112.
283112
First experiments on the synthesis of 283112 produced a SF activity with half-life ~5 min. This activity was also observed from the alpha decay of 287114. More recently 283112 has been observed to undergo 9.52 MeV alpha decay and SF with a half-life of 3.9 s. These results suggest the assignment of the initial activity to an isomeric level in 283112 and the latter confirmed activity to the ground state. Further research is required.
Retracted isotopes
281112
In the claimed synthesis of 293118 in 1999 (see ununoctium) the isotope 281112 was idenitified as decaying by emission of a 10.68 MeV alpha particle with half-life 0.90 ms. The claim was retracted in 2001 and hence this ununbium isotope is currently unknown or unconfirmed.