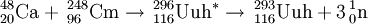Ununhexium
2008/9 Schools Wikipedia Selection. Related subjects: Chemical elements
|
|||||||||||||||||||||||||||||||
| General | |||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Name, Symbol, Number | ununhexium, Uuh, 116 | ||||||||||||||||||||||||||||||
| Element category | presumably poor metal | ||||||||||||||||||||||||||||||
| Group, Period, Block | 16, 7, p | ||||||||||||||||||||||||||||||
| Standard atomic weight | [293] g·mol−1 | ||||||||||||||||||||||||||||||
| Electron configuration | perhaps [Rn] 5f14 6d10 7s2 7p4 (guess based on polonium) |
||||||||||||||||||||||||||||||
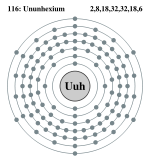
| Electrons per shell | 2, 8, 18, 32, 32, 18, 6 | ||||||||||||||||||||||||||||||
| CAS registry number | 54100-71-9 | ||||||||||||||||||||||||||||||
| Selected isotopes | |||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||
| References | |||||||||||||||||||||||||||||||
Ununhexium (pronounced /ˌjuːnənˈhɛksiəm/ or /ˌʌnənˈhɛksiəm/) is the temporary name of a synthetic superheavy element in the periodic table that has the temporary symbol Uuh and has the atomic number 116.
Four isotopes are currently known with masses 290-293. The most stable is Uuh-293 with a half-life of 63 ms.
Discovery profile
On July 19, 2000, scientists at Dubna (FLNR) detected a single decay from an atom of ununhexium following the irradiation of a Cm-248 target with Ca-48 ions. The results were published in December, 2000. This 10.54 MeV alpha-emitting activity was originally assigned to 292Uuh due to the correlation of the daughter to previously assigned 288Uuq. However, that assignment was later altered to 289Uuq, and hence this activity was correspondingly changed to 293Uuh. Two further atoms were reported by the institute during their second experiment between April-May 2001.
In the same experiment they also detected a decay chain which corresponded to the first observed decay of ununquadium and assigned to 289Uuq. This activity has not been observed again in a repeat of the same reaction. However, its detection in this series of experiments indicates the possibility of the decay of a meta-stable isomer of ununhexium, namely 293m116, or a rare decay branch of the already discovered ground state isomer, in which the first alpha particle was missed. Further research is required to positively assign this activity.
The team repeated the experiment in April-May 2005 and detected 8 atoms of ununhexium. The measured decay data confirmed the assignment of the discovery isotope as 293116. In this run, the team also observed 292116 in the 4n channel for the first time.
Theoretical calculation in a quantum tunneling model supports the experimental data.
The IUPAC/IUPAP Joint Working Party (JWP) are currently assessing the claim of discovery for this element by the Dubna team.
Naming
Current names
The element with Z=116 is historically known as eka-polonium. Ununhexium (Uuh) is a temporary IUPAC systematic element name. Research scientists usually refer to the element simply as element 116 (E116).
Proposed names by Claimants
Claims to the discovery of element 116 have been put forward by Dmitriev of the Dubna team. The JWP will decide to whom the right to suggest a name will be given. The IUPAC have the final say on the official adoption of a name. No name for element 116 has yet been suggested by the Dubna team.
Disallowed names
According to IUPAC rules, names used for previous elements that have ultimately not been adopted are not allowed to be proposed for future use. The table below summarises those names which are probably not allowed to be proposed by the claimant laboratories under the rules.
| Name | Symbol | Reason |
|---|---|---|
| Leosium | Ls | Used for claimed discovery of element 43 |
| Kurchatovium | Ku | Used for claimed discovery of element 104 |
| Flerovium | Fl | Used for claimed discovery of element 102 |
Plausible names
Many speculative names appear in popular literature. The table below lists these names in the case where they obey IUPAC rules and are plausible with regard to the claimant laboratories. Rumored suggestions linked to the claimant laboratories are also included.
| Name | Symbol | Derivation | Comments |
|---|---|---|---|
| Flyorovium | Fl; Fv | Georgy Flyorov, head of the Dubna team | Fl symbol unlikely due to confusion with fluorine (F); flerovium linked to element 118 |
| Butlerovium | Bu; Bv | Aleksandr Butlerov, Russian chemist | unlikely—not a nuclear chemist |
| Rossijium | Ro; Rs | Rossija, transliteration of Russian name for Russia | |
| Taldomskium | Taldomsky, District of Moscow where Dubna lies |
Electronic structure
Ununhexium has 6 full shells, 7 s+5 p+4 d+2 f=18 full subshells, and 116 orbitals:
Bohr model: 2, 8, 18, 32, 32, 18, 6
Quantum mechanical model: 1s22s22p63s23p64s23d10 4p65s24d105p66s24f145d10 6p67s25f146d107p4
Extrapolated chemical properties of eka-polonium
Oxidation states
Element 116 is projected to be the fourth member of the 7p series of non-metals and the heaviest member of group 16 (VIA) in the Periodic Table, below polonium. The group oxidation state of +VI is known for all the members apart from oxygen which lacks available d- orbitals for expansion and is limited to a maximum +II state, exhibited in the fluoride OF2. The +IV is known for sulfur, selenium, tellurium, and polonium, undergoing a shift in stability from reducing for S(IV) and Se(IV) to oxidising in Po(IV). Tellurium(IV) is the most stable for this element. This suggests a decreasing stability for the higher oxidation states as the group is descended and element 116 should portray an oxidising +IV state and a more stable +II state. The lighter members are also known to form a −II state as oxide, sulfide, selenide, and telluride. Polonide formation is nonconfirmed or only transient. The extrapolated electronegativity of ununhexium should eliminate this low oxidation state.
Chemistry
The possible chemistry of element 116 can be extrapolated from that of polonium. It should therefore undergo oxidation to a dioxide, UuhO2, although a trioxide, UuhO3 is plausible, but unlikely. The stability of a +II state should manifest itself in the formation of a simple monoxide, UuhO. Fluorination will likely result in a tetrafluoride, UuhF4 and/or a difluoride, UuhF2. Chlorination and bromination may well stop at the corresponding dihalides, UuhCl2 and UuhBr2. Oxidation by iodine should certainly stop at UuhI2 and may even be inert to this element.
History of synthesis of isotopes by cold fusion
208Pb(82Se,xn)290−x116
In 1995, the team at GSI attempted the synthesis of 290116 as a radiative capture (x=0) product. No atoms were detected providing a cross section limit of 4.8 pb.
History of synthesis of isotopes by hot fusion
238U(54Cr,xn)292−x116
There are sketchy indications that this reaction was attempted by the team at GSI in 2006. There are no published results on the outcome, presumably indicating that no atoms were detected. This is expected from a study of the systematics of cross sections for U-238 targets.
248Cm(48Ca,xn)296−x116 (x=3,4)
The first attempt to synthesise element 116 was performed in 1977 by Ken Hulet and his team at the Lawrence Livermore National Laboratory (LLNL). They were unable to detect any atoms of ununhexium. Yuri Oganessian and his team at the Flerov Laboratory of Nuclear Reactions (FLNR) subsequently attempted the reaction in 1978 and were met by failure. In 1985, a joint experiment between Berkeley and Peter Armbruster's team at GSI, the result was again negative with a calculated cross-section limit of 10–100 pb.
In 2000, Russian scientists at Dubna finally succeeded in detecting a single atom of element 116, assigned to the isotope 292116. In 2001, they repeated the reaction and formed a further 2 atoms in a confirmation of their discovery experiment. A third atom was tentatively assigned to 293116 on the basis of a missed parental alpha decay. In April 2004, the team ran the experiment again at higher energy and were able to detect a new decay chain, assigned to 292116. On this basis, the original data were reassigned to 293116. The tentative chain is therefore possibly associated with a rare decay branch of this isotope. In this reaction, 3 further atoms of 293116 were detected.
245Cm(48Ca,xn)293−x116 (x=2,3)
In order to assist in the assignment of isotope mass numbers for ununhexium, in March-May 2003 the Dubna team bombarded a Cm-245 target with Ca-48 ions. They were able to observe two new isotopes, assigned to 291116 and 290116. This experiment was successfully repeated in Feb-March 2005 where 10 atoms were created with identical decay data to those reported in the 2003 experiment.
Synthesis of ununhexium as a decay product
Ununhexium has also been observed in the decay of ununoctium. In October 2006 it was announced that 3 atoms of ununoctium had been detected by the bombardment of californium-249 with calcium-48 ions, which then rapidly decayed into ununhexium.
The observation of 290116 allowed the assignment of the product to 294118 and proved the synthesis of a nucleus with Z=118 (see ununoctium).
Chronology of isotope discovery
| Isotope | Year discovered | Discoverer reaction |
|---|---|---|
| 290Uuh | 2002 | 249Cf(48Ca,3n) |
| 291Uuh | 2003 | 245Cm(48Ca,2n) |
| 292Uuh | 2004 | 248Cm(48Ca,4n) |
| 293Uuh | 2000 | 248Cm(48Ca,3n) |
Yields of isotopes
Hot fusion
The table below provides cross-sections and excitation energies for hot fusion reactions producing ununhexium isotopes directly. Data in bold represent maxima derived from excitation function measurements. + represents an observed exit channel.
| Projectile | Target | CN | 2n | 3n | 4n | 5n |
|---|---|---|---|---|---|---|
| 48Ca | 248Cm | 296Uuh | 1.1 pb, 38.9 MeV | 3.3 pb, 38.9 MeV | ||
| 48Ca | 245Cm | 293Uuh | 0.9 pb, 33.0 MeV | 3.7 pb, 37.9 MeV |
Retracted isotopes
289116
In 1999, researchers at Lawrence Berkeley National Laboratory announced the synthesis of 293118 (see ununoctium), in a paper published in Physical Review Letters. The claimed isotope 289116 decayed by 11.63MeV alpha emission with a halflife of 0.64 ms. The following year, they published a retraction after other researchers were unable to duplicate the results. In June 2002, the director of the lab announced that the original claim of the discovery of these two elements had been based on data fabricated by the principal author Victor Ninov. As such, this ununhexium isotope is currently unknown or deconfirmed.
Future experiments
The team at Dubna are planning to revisit the synthesis in 2008. They will bombard a plutonium-244 target with titanium-50 ions. This experiment will allow them to assess the feasibility of using projectiles with Z>20 required in the synthesis of SHE's with Z>118. There are also plans to repeat the Cm-248 reaction at different projectile energies in order to probe the 2n channel, leading to the new isotope 294116. In addition they hope to complete the excitation function of the 4n channel product, 292116, which will allow them to assess the stabilising effect of the N=184 shell on the yield of evaporation residues.