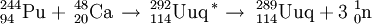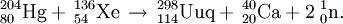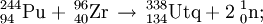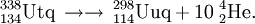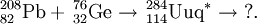Ununquadium
2008/9 Schools Wikipedia Selection. Related subjects: Chemical elements
|
||||||||||||||||||||||||||||||||||
| General | ||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Name, Symbol, Number | ununquadium, Uuq, 114 | |||||||||||||||||||||||||||||||||
| Element category | presumably poor metals | |||||||||||||||||||||||||||||||||
| Group, Period, Block | 14, 7, p | |||||||||||||||||||||||||||||||||
| Appearance | unknown, probably silvery white or metallic gray |
|||||||||||||||||||||||||||||||||
| Standard atomic weight | [289] g·mol−1 | |||||||||||||||||||||||||||||||||
| Electron configuration | perhaps [Rn] 5f14 6d10 7s2 7p2 (guess based on lead) |
|||||||||||||||||||||||||||||||||
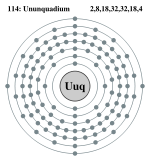
| Electrons per shell | 2, 8, 18, 32, 32, 18, 4 | |||||||||||||||||||||||||||||||||
| Phase | unknown | |||||||||||||||||||||||||||||||||
| CAS registry number | 54085-16-4 | |||||||||||||||||||||||||||||||||
| Selected isotopes | ||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
| References | ||||||||||||||||||||||||||||||||||
Ununquadium (pronounced /ˌjuːnənˈkwɒdiəm/ or /ˌʌnənˈkwɒdiəm/) is the temporary name of a radioactive chemical element in the periodic table that has the temporary symbol Uuq and has the atomic number 114.
First chemistry experiments indicate that element 114 may be the first superheavy to show abnormal noble-gas-like properties due to relativistic effects.
Discovery profile
In December 1998, scientists at Dubna ( Joint Institute for Nuclear Research) in Russia bombarded a Pu-244 target with Ca-48 ions. A single atom of element 114, decaying by 9.67 MeV alpha-emission with a half-life of 30 s, was produced and assigned to 289114. This observation was subsequently published in January 1999. However, the decay chain observed has not been repeated and the exact identity of this activity is unknown although it is possible that it is due to a meta-stable isomer, namely 289m114.
In March 1999, the same team replaced the Pu-244 target with a Pu-242 one in order to produce other isotopes. This time two atoms of element 114 were produced, decaying by 10.29 MeV alpha-emission with a half-life of 5.5 s. They were assigned as 287114. Once again, this activity has not been seen again and it is unclear what nucleus was produced. It is possible that it was a meta-stable isomer, namely 287m114.
The now-confirmed discovery of element 114 was made in June 1999 when the Dubna team repeated the Pu-244 reaction. This time, two atoms of element 114 were produced decaying by emission of 9.82 MeV alpha particles with a half life of 2.6 s.
This activity was initially assigned to 288114 in error, due to the confusion regarding the above observations. Further work in Dec 2002 has allowed a positive reassignment to 289114.
Theoretical estimation of the alpha decay half lives of the isotopes of the element 114 supports the experimental data. The fission-survived isotope 298114 is predicted to have alpha decay half life around 17 days.
Naming
Current Names
The element with Z=114 is historically known as eka-lead. Ununquadium (Uuq) is a temporary IUPAC systematic element name. Research scientists usually refer to the element simply as element 114 (E114).
Proposed names by claimants
Claims to the discovery of element 114 have been put forward by Dmitriev of the Dubna team. The JWP will decide to whom the right to suggest a name will be given. The IUPAC have the final say on the official adoption of a name. The table below gives the names that the teams above have suggested and which can be verified by press interviews.
Disallowed names
According to IUPAC rules, names used for previous elements that have ultimately not been adopted are not allowed to be proposed for future use. The table below summarises those names which are probably not allowed to be proposed by the claimant laboratories under the rules.
| Name | Symbol | Reason |
|---|---|---|
| Russium | Rs | Used for claimed discovery of element 43 |
| Kurchatovium | Ku | Used for claimed discovery of element 104 |
Plausible names
Many speculative names appear in popular literature. The table below lists these names in the case where they obey IUPAC rules and are plausible with regard to the claimant laboratories. Rumored suggestions linked to the claimant laboratories are also included.
| Name | Synbol | Derivation | Comments |
|---|---|---|---|
| Atlantisium | An | Atlantis, reference to fabled island of stability | |
| Lazarevium | Lz | Yuri Lazarev, late former leader of the Dubna team | |
| Oganessium | Og | Yuri Oganessian, leader of the discovery Dubna team |
Electronic structure
Ununquadium has 6 full shells, 7 s+5 p+4 d+2 f=18 full subshells, and 114 orbitals:
Bohr model: 2, 8, 18, 32, 32, 18, 4
Quantum mechanical model: 1s22s22p63s23p64s23d10 4p65s24d105p66s24f145d10 6p67s25f146d107p2
Extrapolated chemical properties of eka-lead
Oxidation states
Element 114 is projected to be the second member of the 7p series of non-metals and the heaviest member of group 14 (IVA) in the Periodic Table, below lead. Each of the members of this group show the group oxidation state of +IV and the latter members have an increasing +II chemistry due to the onset of the "inert pair effect". Tin represents the point at which the stability of the +II and +IV states are similar. Lead, the heaviest member, portrays a switch from the +IV state to the +II state. Element 114 should therefore follow this trend and a possess an oxidising +IV state and a stable +II state.
Chemistry
Element 114 should portray eka-lead chemical properties and should therefore form a monoxide, UuqO, and dihalides, UuqF2, UuqCl2, UuqBr2, and UuqI2. If the +IV state is accessible, it is likely that it is only possible in the oxide, UuqO2, and fluoride, UutF4. It may also show a mixed oxide, Uuq3O4, analogous to Pb3O4.
Some studies also suggest that the chemical behaviour of element 114 might in fact be closer to that of the noble gas radon, than to that of lead.
Physical properties
A summary of the expected properties of element 114 are given in the table below:
| Config. | Oxidation State | First IE | Density | Melting Point | Boiling Point | Hydrides | Fluorides | Chlorides |
|---|---|---|---|---|---|---|---|---|
| 7p27s2 | +2 | 8.5 eV | 14 g/cm3 | 67 °C | 147 °C | H4Uuq | UuqF2 | UuqCl2 |
Experimental chemistry
Atomic gas phase
Two experiments were performed in April-May 2007 in a joint FLNR-PSI collaboration aiming to study the chemistry of element 112. The first experiment involved the reaction 242Pu(48Ca,3n)287114 and the second the reaction 244Pu(48Ca,4n)288114. The adsorption properties of the resultant atoms on a gold surface were compared with those of radon. The first experiment allowed detection of 3 atoms of 283112 (see ununbium) but also seemingly detected 1 atom of 287114. This result was a surprise given the transport time of the product atoms is ~2 s, so element 114 atoms should decay before adsorption. In the second reaction, 2 atoms of 288114 and possibly 1 atom of 289114 were detected. Two of the three atoms portrayed adsorption characteristics associated with a volatile, noble-gas-like element, which has been suggested but is not predicted by more recent calculations. Further experiments will be performed in 2008 to confirm this important result. These experiments did however provide independent confirmation for the discovery of elements 112, 114, and 116 via comparison with published decay data.
History of synthesis of isotopes by cold fusion
208Pb(76Ge,xn)284−x114
The first attempt to synthesise element 114 in cold fusion reactions was performed at GANIL, France in 2003. No atoms were detected providing a yield limit of 1.2 pb.
History of synthesis of isotopes by hot fusion
244Pu(48Ca,xn)292−x114 (x=3,4,5)
The first experiments on the synthesis of element 114 were performed by the team in Dubna in November 1998. They were able to detect a single, long decay chain, assigned to 289114. The reaction was repeated in 1999 and a further 2 atoms of element 114 were detected. The products were assigned to 288114. The team further studied the reaction in 2002. During the measurement of the 3n, 4n, and 5n neutron evaporation excitation functions they were able to detect 3 atoms of 289114, 12 atoms of the new isotope 288114, and 1 atom of the new isotope 287114. Based on these results, the first atom to be detected was tentatively reassigned to 290114 or 289m114, whilst the two subsequent atoms were reassigned to 289114 and therefore belong to the unofficial discovery experiment. In an attempt to study the chemistry of element 112 as the isotope 285112, this reaction was repeated in April 2007. Surprisingly, a PSI-FLNR directly detected 2 atoms of 288114 forming the basis for the first chemical studies of element 114.
242Pu(48Ca,xn)290−x114 (x=2,3,4)
The team at Dubna first studied this reaction in March-April 1999 and detected two atoms of element 114, assigned to 287114. The reaction was repeated in September 2003 in order to attempt to confirm the decay data for 287114 and 283112 since conflicting data for 283112 had been collected (see ununbium). The Russian scientists were able to measure decay data for 288114,287114 and the new isotope 286114 from the measurement of the 2n, 3n, and 4n excitation functions. In April 2006, a PSI-FLNR collaboration used the reaction to determine the first chemical properties of element 112 by producing 283112 as an overshoot product. In a confirmatory experiment in April 2007, the team were able to detect 287114 directly and therefore measure some initial data on the atomic chemical properties of element 114.
Synthesis of isotopes as decay products
The isotopes of ununquadium have also been observed in the decay of elements 116 and 118 (see ununoctium for decay chain).
| Evaporation residue | Observed Uuq isotope |
|---|---|
| 293116 | 289114 |
| 292116 | 288114 |
| 291116 | 287114 |
| 294118, 290116 | 286114 |
Chronology of isotope discovery
| Isotope | Year discovered | Discoverer reaction |
|---|---|---|
| 286Uuq | 2002 | 249Cf(48Ca,3n) |
| 287Uuq | 2002 | 244Pu(48Ca,5n) |
| 288Uuq | 2002 | 244Pu(48Ca,4n) |
| 289Uuq | 1998?, 1999 | 244Pu(48Ca,3n) |
Yields of isotopes
The tables below provide cross-sections and excitation energies for cold fusion reactions producing ununquadium isotopes directly. Data in bold represent maxima derived from excitation function measurements. + represents an observed exit channel.
Cold fusion
| Projectile | Target | CN | 1n | 2n | 3n |
|---|---|---|---|---|---|
| 76Ge | 208Pb | 284Uuq | < 1.2 pb |
Hot fusion
| Projectile | Target | CN | 2n | 3n | 4n | 5n |
|---|---|---|---|---|---|---|
| 48Ca | 242Pu | 290Uuq | 0.5 pb, 32.5 MeV | 3.6 pb, 40.0 MeV | 4.5 pb, 40.0 MeV | <1.4 pb , 45.0 MeV |
| 48Ca | 244Pu | 292Uuq | 1.7 pb, 40.0 MeV | 5.3 pb, 40.0 MeV | 1.1 pb, 52.0 MeV |
Isomerism in ununquadium isotopes
289114
In the first claimed synthesis of element 114, an isotope assigned as 289114 decayed by emitting a 9.71 MeV alpha particle with a lifetime of 30 seconds. This activity was not observed in repetitions of the direct synthesis of this isotope. However, in a single case from the synthesis of 293116, a decay chain was measured starting with the emission of a 9.63 MeV alpha particle with a lifetime of 2.7 minutes. All subsequent decays were very similar to that observed from 289114, presuming that the parent decay was missed. This strongly suggests that the activity should be assigned to an isomeric level. The absence of the activity in recent experiments indicates that the yield of the isomer is ~20% compared to the supposed ground state and that the observation in the first experiment was a fortunate (or not as the case history indicates). Further research is required to resolve these issues.
287114
In a manner similar to those for 289114, first experiments with a 242Pu target identified an isotope 287114 decaying by emission of a 10.29 MeV alpha particle with a lifetime of 5.5 seconds. The daughter spontaneously fissioned with a lifetime in accord with the previous synthesis of 283112. Both these acitivities have not been observed since (see ununbium). However, the correlation suggests that the results are not random and are possible due to the formation of isomers whose yield is obviously dependent on production methods. Further research is required to unravel these discrepancies.
Retracted isotopes
285114
In the claimed synthesis of 293118 in 1999, the isotope 285114 was identified as decaying by 11.35MeV alpha emission with a half-life of 0.58 ms. The claim was retracted in 2001 and hence this ununquadium isotope is currently unknown or unconfirmed.
In search for the island of stability: 298114
According to macroscopic-microscopic (MM) theory, Z=114 is the next spherical magic number. This means that such nuclei are spherical in their ground state and should have high, wide fission barriers to deformation and hence long SF partial half-lives.
In the region of Z=114, MM theory indicates that N=184 is the next spherical neutron magic number and puts forward the nucleus 298114 as a strong candidate for the next spherical doubly magic nucleus, after 208Pb (Z=82, N=126). 298114 is taken to be at the centre of a hypothetical ‘ island of stability’. However, other calculations using relativistic mean field (RMF) theory propose Z=120, 122, and 126 as alternative proton magic numbers depending upon the chosen set of parameters. It is possible that rather than a peak at a specific proton shell, there exists a plateau of proton shell effects from Z=114–126.
It should be noted that calculations suggest that the minimum of the shell-correction energy and hence the highest fission barrier exists for 297115, caused by pairing effects. Due to the expected high fission barriers, any nucleus within this island of stability will exclusively decay by alpha-particle emission and as such the nucleus with the longest half-life is predicted to be 298114. The expected half-life is unlikely to reach values higher than about 10 minutes, unless the N=184 neutron shell proves to be more stabilising than predicted, for which there exists some evidence. In addition, 297114 may have an even-longer half-life due to the effect of the odd neutron, creating transitions between similar Nilsson levels with lower Qalpha values.
In either case, an island of stability does not represent nuclei with the longest half-lives but those which are significantly stabilised against fission by closed-shell effects.
Evidence for Z=114 closed proton shell
Whilst evidence for closed neutron shells can be deemed directly from the systematic variation of Qalpha values for ground-state to ground-state transitions, evidence for closed proton shells comes from (partial) spontaneous fission half-lives. Such data can sometimes be difficult to extract due to low production rates and weak SF branching. In the case of Z=114, evidence for the effect of this proposed closed shell comes from the comparison between the nuclei pairings 282112 (TSF1/2 = 0.8 ms) and 286114 (TSF1/2 = 130 ms), and 284112 (TSF = 97 ms) and 288114 (TSF >800 ms). Further evidence would come from the measurement of partial SF half-lives of nuclei with Z>114, such as 290116 and 292118 (both N=174 isotones). The extraction of Z=114 effects is complicated by the presence of a dominating N=184 effect in this region.
Difficulty in synthesis
The direct synthesis of ununquadium-298 by a fusion-evaporation pathway is impossible since no known combination of target and projectile can provide 184 neutrons in the compound nucleus.
It has been suggested that such a neutron-rich isotope can be formed by the quasi-fission of a massive nucleus. Such nuclei tend to fission with the formation of isotopes close to the closed shells Z=20/N=20 (40Ca), Z=50/N=82 (132Sn)or Z=82/N=126 (208Pb/209Bi). If Z=114 does represent a closed shell, then the reaction below may represent a method of synthesis:
It is also possible that 298114 can be synthesised by the alpha decay of a massive nucleus. Such a method would depend highly on the SF stability of such nuclei, since the alpha half-lives are expected to be very short. The yields for such reactions will most likely be extremely small. One such reaction is
Future experiments
The team at RIKEN are planning to study the reaction