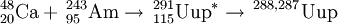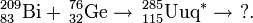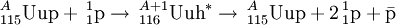Ununpentium
2008/9 Schools Wikipedia Selection. Related subjects: Chemical elements
|
|||||||||||||||||||
| General | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Name, Symbol, Number | ununpentium, Uup, 115 | ||||||||||||||||||
| Element category | presumably poor metals | ||||||||||||||||||
| Group, Period, Block | 15, 7, p | ||||||||||||||||||
| Standard atomic weight | [288] g·mol−1 | ||||||||||||||||||
| Electron configuration | perhaps [Rn] 5f14 6d10 7s2 7p3 (guess based on bismuth) |
||||||||||||||||||
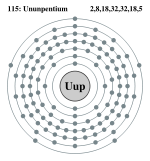
| Electrons per shell | 2, 8, 18, 32, 32, 18, 5 | ||||||||||||||||||
| CAS registry number | 54085-64-2 | ||||||||||||||||||
| Selected isotopes | |||||||||||||||||||
|
|||||||||||||||||||
| References | |||||||||||||||||||
Ununpentium (pronounced /ˌjuːnənˈpɛntiəm/ or /ˌʌnənˈpɛntiəm/) is the temporary name of a synthetic superheavy element in the periodic table that has the temporary symbol Uup and has the atomic number 115.
Two isotopes are currently known, Uup-287 and Uup-288.
Element 115 also falls in the centre of the theoretical island of stability. The most stable isotope of ununpentium is predicted to be Uup-299, containing the " magic number" of 184 neutrons. The most neutron rich isotope to date is Uup-288, which contains only 173 neutrons.
Discovery profile
On February 2, 2004, synthesis of ununpentium was reported in Physical Review C by a team composed of Russian scientists at the Joint Institute for Nuclear Research in Dubna, and American scientists at the Lawrence Livermore National Laboratory. The team reported that they bombarded americium-243 with calcium-48 ions to produce four atoms of ununpentium. These atoms, they report, decayed by emission of alpha-particles to ununtrium in approximately 100 milliseconds.
The Dubna-Livermore collaboration has strengthened their claim for the discovery of ununpentium by conducting chemical experiments on the decay daughter 268Db. In experiments in June 2004 and December 2005, the Dubnium isotope was successfully identified by milking the Db fraction and measuring any SF activities. Both the halflife and decay mode were confirmed for the proposed 268Db which lends support to the assignment of Z=115 to the parent nuclei.
Naming
Current names
The element with Z=115 is historically known as eka-bismuth. Ununpentium (Uup) is a temporary IUPAC systematic element name. Research scientists usually refer to the element simply as element 115 (E115).
Proposed names by claimants
Claims to the discovery of element 115 have been put forward by Dmitriev of the Dubna team. The Joint Working Party will decide to whom the right to suggest a name will be given. The IUPAC have the final say on the official adoption of a name. The table below gives the names that the teams above have suggested and which can be verified by press interviews.
Disallowed names
According to IUPAC rules, names used for previous elements that have ultimately not been adopted are not allowed to be proposed for future use. The table below summarises those names which are probably not allowed to be proposed by the claimant laboratories under the rules.
| Name | Symbol | Reason |
|---|---|---|
| Russium | Rs | Used for claimed discovery of element 43 |
| Kurchatovium | Ku | Used for claimed discovery of element 104 |
Plausible names
Many speculative names appear in popular literature. The table below lists these names in the case where they obey IUPAC rules and are plausible with regard to the claimant laboratories. Rumoured suggestions (*) linked to the claimant laboratories are also included.
| Suggested Name | Suggested Synbol | Derivation | Comments |
|---|---|---|---|
| Langevenium* | Ln | Langevin, prominent French nuclear physicist | Russian team unlikely to use a French physicist ? |
Electronic structure
Ununpentium has 6 full shells, 7 s+5 p+4 d+2 f=18 full subshells, and 115 orbitals:
Bohr model: 2, 8, 18, 32, 32, 18, 5
Quantum mechanical model: 1s22s22p63s23p64s23d10 4p65s24d105p66s24f145d10 6p67s25f146d107p3
Extrapolated chemical properties of eka-bismuth
Oxidation states
Element 115 is projected to be the third member of the 7p series of non-metals and the heaviest member of group 15 (VA) in the Periodic Table, below bismuth. In this group, each member is known to portray the group oxidation state of +V but with differing stability. For nitrogen, the +V state is very difficult to achieve due to the lack of low-lying d- orbitals and the inability of the small nitrogen atom to accommodate five ligands. The +V state is well represented for phosphorus, arsenic, and antimony. However, for bismuth it is rare due to the reluctance of the 6s2 electron to participate in bonding. This effect is known as the "inert pair effect" and is commonly linked to relativistic stabilisation of the 6s-orbitals. It is expected that element 115 will continue this trend and portray only +III and +I oxidation states. Nitrogen(I) and bismuth(I) are known but rare and Uup(I) is likely to show some unique properties.
Chemistry
It is expected that the chemistry of ununpentium will be related to its lighter homologue bismuth. In this regard it is expected to undergo oxidation only as far as the trioxide Uup2O3. Oxidation with the more reactive halogens should form the trihalides, such as UupF3 and UupCl3. The less-oxidising, heavier halogens, may well only be able to promote the formation of the monohalides, UupBr and UupI.
History of synthesis of isotopes by hot fusion
238U(51V,xn)289−x115
There are strong indications that this reaction was performed in late 2004 as part of a uranium(IV) fluoride target test at the GSI. No reports have been published suggesting that no products atoms were detected, as anticipated by the team.
243Am(48Ca,xn)291−x115 (x=3,4)
This reaction was first performed by the team in Dubna in July-August 2003. In two separate runs they were able to detect 3 atoms of 288115 and a single atom of 287115. The reaction was studied further in June 2004 in an attempt to isolate the descendant 268Db from the 288115 decay chain. After chemical separation of a +4/+5 fraction, 15 SF decays were measured with a lifetime consistent with 268Db. In order to prove that the decays were from dubnium-268, the team repeated the reaction in August 2005 and separated the +4 and +5 fractions and further separated the +5 fractions into tantalum-like and niobium-like ones. Five SF activities were observed, all occurring in the +5 fractions and none in the tantalum-like fractions, proving that the product was indeed isotopes of dubnium.
Chronology of isotope discovery
| Isotope | Year discovered | Discoverer reaction |
|---|---|---|
| 287Uup | 2003 | 243Am(48Ca,4n) |
| 288Uup | 2003 | 243Am(48Ca,3n) |
Yields of isotopes
Hot fusion
The table below provides cross-sections and excitation energies for hot fusion reactions producing ununpentium isotopes directly. Data in bold represent maxima derived from excitation function measurements. + represents an observed exit channel.
| Projectile | Target | CN | 2n | 3n | 4n | 5n |
|---|---|---|---|---|---|---|
| 48Ca | 243Am | 291Uup | 3.7 pb, 39.0 MeV | 0.9 pb, 44.4 MeV |
Future experiments
The team at RIKEN are planning to study the reaction
As a primary next-goal for the Dubna team, they are planning to examine to products of the 243Am + 48Ca using mass spectrometry in their state-of-the-art MASHA machine. They will attempt to isolate the dubnium products, convert them chemically into a volatile compound, most likely 268DbCl5, and measure the mass directly.
In popular culture
UFO propulsion
Element 115 was first mentioned in the 1980s in association with UFO conspiracy theories. The most popular account of element 115 is from Bob Lazar, a scientist who claims to have worked on a top-secret, alien craft reverse-engineering program at a site known as S-4 near Groom Lake, Nevada. Many popular culture websites exist which discuss and critique the claims surrounding the element and the field.
Bob Lazar claims that Element 115 exists as a stable isotope. It's used as a fuel via nuclear reactions with proton projectiles in which antiprotons are created. These are then collected and channeled into an annihilation chamber where they react with protons to form gamma rays and lots of energy. This enormous power is used to fantastically multiply the minuscule Gravity-A wave also produced by the onboard Element 115 reactor.
Gravity-A is understood to exist only between atomic nuclear particles and is vastly more powerful than the Gravity-B with which we are all familiar from our own everyday experience. Element 115 is unusual in that the Gravity-A effect extends just beyond the perimeter of the Element 115 atom and with very advanced technology can be tapped and amplified and then directed via gravity distortion devices to produce a kind of "propulsive" effect for the alien spacecraft. What is supposedly happening is that the craft is creating a large enough space-time distortion along its direction of orientation that it essentially "falls downhill" through the artificially generated gravity corridor.
Science-based critique of claim
Stable E115
There is much debate regarding the possibility of a stable isotope of element 115. The element is expected to lie within the island of stability and recent results have confirmed its existence (see ununquadium). The recent synthesis of two isotopes of ununpentium has been taken to suggest that the existence of a stable isotope is unlikely. However, many critics fail to appreciate the nature of closed magic shells. In particular, most people focus solely on the much talked about N=184 shell. It should be pointed out that this is not the only closed magic neutron shell. Calculations have indicated a closed deformed magic shell at N=196 and a spherical closed shell at N=228. These calculations in conjunction with expected trends strongly imply that more stable isotopes of element 114 are possibly 310114 (N=196) and most likely 342114 (N=226). Taking hindrance of fission by odd particles into account, the most stable isotope of element 115 is probably 345115 and definitely not 299115. Such nuclei also have much more favourable N/Z values and lie closer to the classical stability line.298114 is in fact relatively neutron deficient for a Z=114 nucleus (N/Z 2.61 c.f. 2.60 for 244Pu) using a classical liquid-drop approach. Using the trends in Qalpha vs N from recent calculations, extrapolations indicate that Qalpha should fall on approach to the N=228 shell and Qalpha may reach a value of ~5.8 MeV for 342114. This provides a halflife of 1.83x1010 years (using Viola-Seaborg equation) and can be taken to be 'stable', like uranium.
Antiproton synthesis
The claimed method of synthesis of the antiprotons is known from the study of cosmic rays. Interactions between protons and nuclei lead to the formation of so-called secondary antiprotons which could be stored and used. The reaction would be inferred to be:
Annihilation
The study of the proton-antiproton annihilation reaction is well-known and is currently the subject of much research.
Levitation effects
Levitation in materials is caused by diamagnetic properties. Bismuth is known to be the strongest of all diamagnetic materials on Earth and hence element 115, as its heavier homolog, may well have even stronger diamagnetic attributes. Recent research on the synthesis of element 113 has highlighted the effect of the structure of the bismuth nucleus on reactions and as such it should be prone to manipulation using electrorotation. These levitation effects though do not have anything to do with gravity manipulation, as they are purely electromagnetic in nature. (diamagnetism is a form of magnetism, not gravity) That certain elements can have anomalous gravitational effects is unproven.
Summary
Some of the claimed properties of element 115 may have some verification from mainstream science, either as hard experimental evidence or in modern theoretical calculations. Others are either lacking or are the result of simple misunderstanding as in the case of the erroneously attributed claims of gravity manipulation properties.
Other
This element was used as fuel for a time machine in the Science fiction show Seven Days. It is also the most desired resource in the X-Com series of tactical computer games.