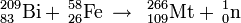Meitnerium
2008/9 Schools Wikipedia Selection. Related subjects: Chemical elements
|
|||||||||||||||||||||||||||||||||||||||||||||||||
| General | |||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Name, Symbol, Number | meitnerium, Mt, 109 | ||||||||||||||||||||||||||||||||||||||||||||||||
| Chemical series | transition metals | ||||||||||||||||||||||||||||||||||||||||||||||||
| Group, Period, Block | 9, 7, d | ||||||||||||||||||||||||||||||||||||||||||||||||
| Appearance | unknown, probably silvery white or metallic gray |
||||||||||||||||||||||||||||||||||||||||||||||||
| Standard atomic weight | [270] g·mol−1 | ||||||||||||||||||||||||||||||||||||||||||||||||
| Electron configuration | perhaps [Rn] 5f14 6d7 7s2 (guess based on iridium) |
||||||||||||||||||||||||||||||||||||||||||||||||
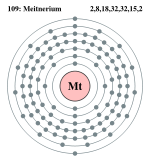
| Electrons per shell | 2, 8, 18, 32, 32, 15, 2 | ||||||||||||||||||||||||||||||||||||||||||||||||
| Phase | presumably a solid | ||||||||||||||||||||||||||||||||||||||||||||||||
| CAS registry number | 54038-01-6 | ||||||||||||||||||||||||||||||||||||||||||||||||
| Selected isotopes | |||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||
| References | |||||||||||||||||||||||||||||||||||||||||||||||||
Meitnerium (pronounced /maɪtˈnɜriəm/) is a chemical element in the periodic table that has the symbol Mt and atomic number 109.
Mt is a synthetic element whose most stable known isotope, Mt-278, has a half-life of half an hour.
Discovery profile
Meitnerium was first synthesized on August 29, 1982 by a German research team led by Peter Armbruster and Gottfried Münzenberg at the Institute for Heavy Ion Research (Gesellschaft für Schwerionenforschung) in Darmstadt. The team bombarded a target of bismuth-209 with accelerated nuclei of iron-58 and detected a single atom of the isotope meitnerium-266:
Proposed names
Historically, element 109 has been referred to as eka-iridium.
The name meitnerium (Mt) was suggested in honour of the Austrian physicist and mathematician Lise Meitner, but there was an element naming controversy as to what the elements from 101 to 109 were to be called; thus IUPAC adopted unnilennium (/ˌjuːnɪˈlɛniəm/ or /ˌʌːnɪˈlɛniəm/, symbol Une) as a temporary, systematic element name. In 1997, however, the dispute was resolved and the current name was adopted.
Electronic structure
Meitnerium is element 109 in the Periodic Table. The two forms of the projected electronic structure are:
Bohr model: 2, 8, 18, 32, 32, 15, 2
Quantum mechanical model: 1s22s22p63s23p64s23d10 4p65s24d105p66s24f145d10 6p67s25f146d7
Extrapolated chemical properties of eka-iridium/dvi-rhodium
Oxidation states
Element 109 is projected to be the sixth member of the 6d series of transition metals and the heaviest member of group 9 in the Periodic Table, below cobalt, rhodium and iridium. This group of transition metals are the first to show lower oxidation states and the +IX state is not known. The latter two members of the group show a maximum oxidation state of +VI, whilst the most stable states are +IV and +III for iridium and +III for rhodium. Meitnerium is therefore expected to form a stable +III state but may also portray stable +IV and +VI states.
Chemistry
The +VI state is known only for the fluorides which are formed by direct reaction. Therefore, meitnerium should form a hexafluoride, MtF6. This fluoride is expected to be more stable than iridium(VI) fluoride, as the +VI state becomes more stable as the group is descended. In combination with oxygen, rhodium forms Rh2O3 whilst iridium is oxidised to the +IV state in IrO2. Meitnerium may therefore show a dioxide MtO2 if eka-iridium reactivity is shown. The +III state is common in the trihalides (not fluorides) formed by direct reaction with halogens. Meitnerium should therefore form MtCl3, MtBr3 and MtI3 in an analogous manner to iridium.
History of synthesis of isotopes in cold fusion
209Bi(58Fe,xn)267-xMt (x=1)
The first success in this reaction was in 1982 by the GSI team in their discovery experiment with the identification of a single atom of 266Mt in the 1n neutron evaporation channel. The GSI team used the parent-daughter correlation technique. After an initial failure in 1983, in 1985 the team at the FLNR, Dubna, observed alpha decays from the descendant 246Cf indicating the formation of meitnerium. The GSI synthesised a further 2 atoms of 266Mt in 1988 and continued in 1997 with the detection of 12 atoms during the measurement of the 1n excitation function.
208Pb(59Co,xn)267-xMt (x=1)
This reaction was first studied in 1985 by the team in Dubna. They were able to detect the alpha decay of the descendant 246Cf nuclei indicating the formation of meitnerium atoms. In 2007, in a continuation of their study of the effect of odd-Z projectiles on yields of evaporation residues in cold fusion reactions, the team at LBNL synthesised 266Mt and were able to correlate the decay with known daughters.
181Ta(86Kr,xn)267-xMt
There are indications that this cold fusion reaction using a tantalum target was attempted in August 2001 at the GSI. No details can be found suggesting that no atoms of meitnerium were detected.
History of synthesis by hot fusion reactions
238U(37Cl,xn)275-xMt
In 2002-2003, the team at LBNL attempted the above reaction in order to search for the isotope 271Mt with hope that it may be sufficiently stable to allow a first study of the chemical properties of meitnerium. Unfortunately, no atoms were detected and a cross section limit of 1.5 pb was measured for the 4n channel at the projectile energy used.
254Es(22Ne,xn)276-xMt
Attempts to produce long-living isotopes of meitnerium were first performed by Ken Hulet at the Lawrence Livermore National Laboratory (LLNL) in 1988 using the asymmetric hot fusion reaction above. They were unable to detect any product atoms and established a cross section limit of 1 nb.
Synthesis of isotopes as decay products
Isotopes of meitnerium have also been detected in the decay of heavier elements. Observations to date are shown in the table below:
| Evaporation Residue | Observed Mt isotope |
|---|---|
| 288115 | 276Mt |
| 287115 | 275Mt |
| 282113 | 274Mt |
| 278113 | 270Mt |
| 272Rg | 268Mt |
Chronology of isotope discovery
| Isotope | Year discovered | discovery reaction |
|---|---|---|
| 266Mt | 1982 | 209Bi(58Fe,n) |
| 267Mt | unknown | |
| 268Mt | 1994 | 209Bi(64Ni,n) |
| 269Mt | unknown | |
| 270Mt | 2004 | 209Bi(70Zn,n) |
| 271Mt | unknown | |
| 272Mt | unknown | |
| 273Mt | unknown | |
| 274Mt | 2006 | 237Np(48Ca,3n) |
| 275Mt | 2003 | 243Am(48Ca,4n) |
| 276Mt | 2003 | 243Am(48Ca,3n) |
Chemical yields of isotopes
Cold Fusion
The table below provides cross-sections and excitation energies for cold fusion reactions producing meitnerium isotopes directly. Data in bold represents maxima derived from excitation function measurements. + represents an observed exit channel.
| Projectile | Target | CN | 1n | 2n | 3n |
|---|---|---|---|---|---|
| 58Fe | 209Bi | 267Mt | 7.5 pb | ||
| 59Co | 208Pb | 267Mt | 2.6 pb , 14.9 MeV |
Isomerism in meitnerium nuclides
270Mt
Two atoms of 270Mt have been identified in the decay chains of 278113. The two decays have very different lifetimes and decay energiesand are also produced from two apparently different isomers in 274Rg. The first isomer decays by emission of an 10.03 MeV alpha particle with a lifetime 7.2 ms. The other decays by emitting an alpha particle with a lifetime of 1.63 s. An assignment to specific levels is not possible with the limited data available. Further research is required.
268Mt
The alpha decay spectrum for 268Mt appears to be complicated from the results of several experiments. Alpha lines of 10.28,10.22 ans 10.10 MeV have been observed. Half-lives of 42 ms, 21 ms and 102 ms have been determined. The long-lived decay is associated with alpha particles of energy 10.10 MeV and must be assigned to an isomeric level. The discrepancy between the other two half-lives has yet to be resolved. An assignment to specific levels is not possible with the data available and further research is required.