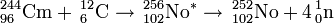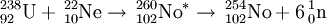Nobelium
2008/9 Schools Wikipedia Selection. Related subjects: Chemical elements
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| General | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Name, Symbol, Number | nobelium, No, 102 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chemical series | actinides | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Group, Period, Block | n/a, 7, f | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Appearance | unknown, probably silvery white or metallic gray |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Standard atomic weight | [259] g·mol−1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Electron configuration | [Rn] 5f14 7s2 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
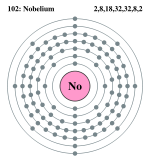
| Electrons per shell | 2, 8, 18, 32, 32, 8, 2 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Physical properties | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Phase | solid | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Atomic properties | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Oxidation states | 2, 3 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Electronegativity | (Pauling scale) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Ionization energies | 1st: 641.6 kJ/mol | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 2nd: 1254.3 kJ/mol | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 3rd: 2605.1 kJ/mol | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Miscellaneous | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| CAS registry number | 10028-14-5 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Selected isotopes | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| References | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Nobelium (pronounced /noʊˈbɛliəm/ or /noʊˈbiːliəm/) is a synthetic element with the symbol No and atomic number 102.
It was first correctly identified in 1966 by scientists at the Flerov Laboratory of Nuclear Reactions in Dubna, Russia. Little is known about the element but limited chemical experiments have shown that it forms a stable divalent ion in solution as well as the predicted trivalent ion that is associated with its presence as one of the actinoids.
Discovery profile
The discovery of element 102 was first announced by physicists at the Nobel Institute in Sweden in 1957. The team reported that they created an isotope with a half-life of 10 minutes, decaying by emission of an 8.5 MeV alpha particle, after bombarding 244Cm with 13C nuclei. The activity was assigned to 251102 or 253102. The scientists proposed the name nobelium (No) for the new element. Later they retracted their claim and associated the activity to background effects.
The synthesis of element 102 was then claimed in April 1958 at the University of California, Berkeley by Albert Ghiorso, Glenn T. Seaborg, John R. Walton and Torbjørn Sikkeland. The team used the new heavy-ion linear accelerator (HILAC) to bombard a curium target (95% 244Cm and 5% 246Cm) with 13C and 12C ions. They were unable to confirm the 8.5 MeV activity claimed by the Swedes but were instead able to detect decays from 250Fm, supposedly the daughter of 254102, which had an apparent half-life of ~3 s. In 1959 the team continued their studies and claimed that they were able to produce an isotope that decayed predominantly by emission of an 8.3 MeV alpha particle, with a half-life of 3 s with an associated 30% spontaneous fission branch. The activity was initially assigned to 254No but later changed to 252No. The Berkeley team decided to adopt the name nobelium for the element.
Further work in 1961 on the attempted synthesis of element 103 (see lawrencium) produced evidence for a Z=102 alpha activity decaying by emission of an 8.2 MeV particle with a half-life of 15 s, and assigned to 255No.
Following initial work between 1958-1964, in 1966, a team at the Flerov Laboratory of Nuclear Reactions (FLNR) reported that they had been able to detect 250Fm from the decay of a parent nucleus (254No) with a half-life of ~50s, in contradiction to the Berkeley claim. Furthermore, they were able to show that the parent decayed by emission of 8.1 MeV alpha particles with a half-life of ~35 s.
In 1969, the Dubna team carried out chemical experiments on element 102 and concluded that it behaved as the heavier homologue of Ytterbium. The Russian scientists proposed the name joliotium (Jo) for the new element.
Later work in 1967 at Berkeley and 1971 at Oak Ridge fully confirmed the discovery of element 102 and clarified earlier observations.
In 1992, the IUPAC-IUPAP Transfermium Working Group (TWG) assessed the claims of discovery and concluded that only the Dubna work from 1966 correctly detected and assigned decays to Z=102 nuclei at the time. The Dubna team are therefore officially recognised as the discoverers of nobelium although it is possible that it was detected at Berkeley in 1959.
Naming
Element 102 was first named nobelium (No) by its claimed discoverers in 1957 by scientists at the Nobel Institute in Sweden. The name was later adopted by Berkeley scientists who claimed its discovery in 1959.
The International Union of Pure and Applied Chemistry ( IUPAC) officially recognised the name nobelium following the Berkeley results.
In 1969, Russian scientists working in Dubna disputed the claims of these groups and suggested the name joliotium (Jo), in recognition of the work of Frédéric Joliot-Curie.
In 1992, the TWG recognised the Dubna scientists as the official discoverers and acknowledged that the adoption of nobelium as the official name had been made prematurely.
Subsequently, there are indications that the IUPAC suggested the name flerovium (Fl) for the element in recognition of the Dubna laboratory and the name has been used in the literature in reference to the element.
However, in 1994, and subsequently in 1997, the IUPAC ratified the name nobelium (No) for the element on the basis that it had become entrenched in the literature over the course of 30 years and that Alfred Nobel should be commemorated in this fashion.
Contrary to some suggestions, at no time has element 102 been referred to as unnilbium (/juːˈnɪlbiəm/, symbol Unb) due to the above circumstances.
Electronic structure
Nobelium is element 102 in the Periodic Table. The two forms of the projected electronic structure are:
Bohr model: 2, 8, 18, 32, 32, 8, 2
Quantum mechanical model: 1s22s22p63s23p64s23d10 4p65s24d105p66s24f145d10 6p67s25f14
Physical properties
The appearance of this element is unknown, however it is most likely silvery-white or gray and metallic. If sufficient amounts of nobelium were produced, it would pose a radiation hazard. Some sources quote a melting point of 827oC for nobelium but this cannot be substantiated from an official source and seems implausible regarding the requirements of such a measurement.However, the 1st, 2nd and 3rd ionization energies have been measured. In addition, an electronegativity value of 1.3 is also sometimes quoted. This is most definitely only an estimate since a true value can only be determined using a chemical compound of the element and no such compounds exist for nobelium.
Experimental chemistry
Aqueous phase chemistry
First experiments involving nobelium assumed that it predominantly formed a +III state like earlier actinoids. However, it was later found that nobelium forms a stable +II state in solution, although it can be oxidised to an oxidising +III state. A reduction potential of -1.78 V has been measured for the No3+ ion. The hexaaquanobelium(II) ion has been determined to have an ionic radius of 110 pm.
Summary of compounds and (complex) ions
| Formula | Names(s) |
|---|---|
| [No(H2O)6]3+ | hexaaquanobelium(III) |
| [No(H2O)6]2+ | hexaaquanobelium(II) |
Isotopes
Seventeen radioisotopes of nobelium have been characterized, with the most stable being 259No with a half-life of 58 minutes. Longer half-lives are expected for the as-yet-unknown 261No and 263No. An isomeric level has been found in 253No and K-isomers have been found in 250No, 252No and 254No to date.
History of synthesis of isotopes by cold fusion
208Pb(48Ca,xn)256-xNo (x=1,2,3,4)
This cold fusion reaction was first studied in 1979 at the FLNR. Further work in 1988 at the GSI measured EC and SF branchings in 254No. In 1989, the FLNR used the reaction to measure SF decay characteristics for the two isomers of 254No. The measurement of the 2n excitation function was reported in 2001 by Yuri Oganessian at the FLNR.
Patin et al. at the LBNL reported in 2002 the synthesis of 255-251No in the 1-4n exit channels and measured further decay data for these isotopes.
The reaction has recently been used at the Jyvaskylan Yliopisto Fysiikan Laitos (JYFL) using the RITU set-up to study K-isomerism in 254No. The scientists were able to measure two K-isomers with half-lives of 275 ms and 198 µs, respectively. They were assigned to 8- and 16+ K-isomeric levels.
The reaction was used in 2004-5 at the FLNR to study the spectroscopy of 255-253No. The team were able to confirm an isomeric level in 253No with a half-life of 43.5 µs.
208Pb(44Ca,xn)252-xNo (x=2)
This reaction was studied in 2003 at the FLNR in a study of the spectroscopy of 250No.
207Pb(48Ca,xn)255-xNo (x=2)
The measurement of the 2n excitation function for this reaction was reported in 2001 by Yuri Oganessian and co-workers at the FLNR. The reaction was used in 2004-5 to study the spectroscopy of 253No.
206Pb(48Ca,xn)254-xNo (x=1,2,3,4)
The measurement of the 1-4n excitation functions for this reaction were reported in 2001 by Yuri Oganessian and co-workers at the FLNR. The 2n channel was further studied by the GSI to provide a spectroscopic determination of K-isomerism in 252No. A K-isomer with spin and parity 8- was detected with a half-life of 110 ms.
204Pb(48Ca,xn)252-xNo (x=2)
The measurement of the 2n excitation function for this reaction was reported in 2001 by Yuri Oganessian at the FLNR. They reported a new isotope 250No with a half-life of 36µs. The reaction was used in 2003 to study the spectroscopy of 250No.They were able to observe two spontaneous fission activities with half-lives of 5.6µs and 54µs and assigned to 250No and 249No, respectively. The latter activity was later assigned to a K-isomer in 250No. The reaction was reported in 2006 by Peterson et al. at the Argonne National Laboratory (ANL) in a study of SF in 250No. They detected two activities with half-lives of 3.7µs and 43µs and both assigned to 250No, the latter associated with a K-isomer.
History of synthesis of isotopes by hot fusion
232Th(26Mg,xn)258-xNo (x=4,5,6)
The cross sections for the 4-6n exit channels have been measured for this reaction at the FLNR.
238U(22Ne,xn)260-xNo (x=4,5,6)
This reaction was first studied in 1964 at the FLNR. The team were able to detect decays from 252Fm and 250Fm. The 252Fm activity was associated with an ~8 s half-life and assigned to 256102 from the 4n channel, with a yield of 45 nb. They were also able to detect a 10 s spontaneous fission activity also tentatively assigned to 256102. Further work in 1966 on the reaction examined the detection of 250Fm decay using chemical separation and a parent activity with a half-life of ~50 s was reported and correctly assigned to 254102. They also detected a 10 s spontaneous fission activity tentatively assigned to 256102. The reaction was used in 1969 to study some initial chemistry of nobelium at the FLNR. They determined eka-ytterbium properties, consistent with nobelium as the heavier homologue. In 1970, they were able to study the SF properties of 256No. In 2002, Patin et al. reported the synthesis of 256No from the 4n channel but were unable to detect 257No.
The cross section values for the 4-6n channels have also been studied at the FLNR.
238U(20Ne,xn)258-xNo
This reaction was studied in 1964 at the FLNR. No spontaneous fission activities were observed.
236U(22Ne,xn)258-xNo (x=4,5,6)
The cross sections for the 4-6n exit channels have been measured for this reaction at the FLNR.
235U(22Ne,xn)257-xNo (x=5)
This reaction was studied in 1970 at the FLNR. It was used to study the SF decay properties of 252No.
233U(22Ne,xn)255-xNo
The synthesis of neutron deficient nobelium isotopes was studied in 1975 at the FLNR. In their experiments they observed a 250 µs SF activity which they tentatively assigned to 250No in the 5n exit channel. Later results have not been able to confirm this activity and it is currently unidentified.
242Pu(18O,xn)260-xNo (x=4?)
This reaction was studied in 1966 at the FLNR. The team identified an 8.2 s SF activity tentatively assigned to 256102.
241Pu(16O,xn)257-xNo
This reaction was first studied in 1958 at the FLNR. The team measured ~8.8 MeV alpha particles with a half-life of 30 s and assigned to 253,252,251102. A repeat in 1960 produced 8.9 MeV alpha particles with a half-life of 2-40 s and assigned to 253102 from the 4n channel. Confidence in these results was later diminished.
239Pu(18O,xn)257-xNo (x=5)
This reaction was studied in 1970 at the FLNR in an effort to study the SF decay properties of 252No.
239Pu(16O,xn)255-xNo
This reaction was first studied in 1958 at the FLNR. The team were able to measure ~8.8 MeV alpha particles with a half-life of 30 s and assigned to 253,252,251102. A repeat in 1960 was unsuccessful and it was concluded the first results were probably associated with background effects.
243Am(15N,xn)258-xNo (x=4)
This reaction was studied in 1966 at the FLNR. The team were able to detect 250Fm using chemical techniques and determined an associated half-life significantly higher than the reported 3 s by Berkeley for the supposed parent 254No. Further work later the same year measured 8.1 MeV alpha particles with a half-life of 30-40 s.
243Am(14N,xn)257-xNo
This reaction was studied in 1966 at the FLNR. They were unable to detect the 8.1 MeV alpha particles detected when using a N-15 beam.
241Am(15N,xn)256-xNo (x=4)
The decay properties of 252No were examined in 1977 at Oak Ridge. The team calculated a half-life of 2.3 s and measured a 27% SF branching.
248Cm(18O,αxn)262-xNo (x=3)
The synthesis of the new isotope 259No was reported in 1973 from the LBNL using this reaction.
248Cm(13C,xn)261-xNo (x=3?,4,5)
This reaction was first studied in 1967 at the LBNL. The new isotopes 258No,257No and 256No were detected in the 3-5n channels. The reaction was repeated in 1970 to provide further decay data for 257No.
248Cm(12C,xn)260-xNo (4,5?)
This reaction was studied in 1967 at the LBNL in their seminal study of nobelium isotopes. The reaction was used in 1990 at the LBNL to study the SF of 256No.
246Cm(13C,xn)259-xNo (4?,5?)
This reaction was studied in 1967 at the LBNL in their seminal study of nobelium isotopes.
246Cm(12C,xn)258-xNo (4,5)
This reaction was studied in 1958 by scientists at the LBNL using a 5% 246Cm curium target. They were able to measure 7.43 MeV decays from 250Fm, associated with a 3 s 254No parent activity, resulting from the 4n channel. The 3 s activity was later reassigned to 252No, resulting from reaction with the predominant 244Cm component in the target. It could however not be proved that it was not due to the contaminant 250mFm, unknown at the time. Later work in 1959 produced 8.3 MeV alpha particles with a half-life of 3 s and a 30% SF branch. This was initially assigned to 254No and later reassigned to 252No, resulting from reaction with the 244Cm component in the target. The reaction was restudied in 1967 and activities assigned to 254No and 253No were detected.
244Cm(13C,xn)257-xNo (x=4)
This reaction was first studied in 1957 at the Nobel Institute in Stockholm. The scientists detected 8.5 MeV alpha particles with a half-life of 10 minutes. The activity was assigned to 251No or 253No. The results were later dismissed as background. The reaction was repeated by scientists at the LBNL in 1958 but they were unable to confirm the 8.5 MeV alpha particles. The reaction was further studied in 1967 at the LBNL and an activity assigned to 253No was measured.
244Cm(12C,xn)256-xNo (x=4,5)
This reaction was studied in 1958 by scientists at the LBNL using a 95% 244Cm curium target. They were able to measure 7.43 MeV decays from 250Fm, associated with a 3 s 254No parent activity, resulting from the reaction (246Cm,4n). The 3 s activity was later reassigned to 252No, resulting from reaction (244Cm,4n). It could however not be proved that it was not due to the contaminant 250mFm, unknown at the time. Later work in 1959 produced 8.3 MeV alpha particles with a half-life of 3 s and a 30% SF branch. This was initially assigned to 254No and later reassigned to 252No, resulting from reaction with the 244Cm component in the target. The reaction was restudied in 1967 at the LBNL and an new activity assigned to 251No was measured.
252Cf(12C,αxn)260-xNo (x=3?)
This reaction was studied at the LBNL in 1961 as part of their search for element 104. They detected 8.2 MeV alpha particles with a half-life of 15 s. This activity was assigned to a Z=102 isotope. Later work suggests an assignment to 257No, resulting most likely from the α3n channel with the 252Cf component of the californium target.
252Cf(11B,pxn)262-xNo (x=5?)
This reaction was studied at the LBNL in 1961 as part of their search for element 103. They detected 8.2 MeV alpha particles with a half-life of 15 s. This activity was assigned to a Z=102 isotope. Later work suggests an assignment to 257No, resulting most likely from the p5n channel with the 252Cf component of the californium target.
249Cf(12C,αxn)257-xNo (x=2)
This reaction was first studied in 1970 at the LBNL in a study of 255No. It was studied in 1971 at the Oak Ridge Laboratory. They were able to measure coincident Z=100 K X-rays from 255No, confirming the discovery of the element.
Synthesis of isotopes as decay products
Isotopes of nobelium have also been identified in the decay of heavier elements. Observations to date are summarised in the table below:
| Evaporation Residue | Observed No isotope |
|---|---|
| 262Lr | 262No |
| 269Hs, 265Sg, 261Rf | 257No |
| 267Hs, 263Sg, 259Rf | 255No |
| 254Lr | 254No |
| 261Sg, 257Rf | 253No |
| 264Hs, 260Sg, 256Rf | 252No |
| 255Rf | 251No |
Chronology of isotope discovery
| Isotope | Year discovered | Discovery reaction |
|---|---|---|
| 250Nom | 2001 | 204Pb(48Ca,2n) |
| 250Nog | 2006 | 204Pb(48Ca,2n) |
| 251No | 1967 | 244Cm(12C,5n) |
| 252Nog | 1959 | 244Cm(12C,4n) |
| 252Nom | ~2002 | 206Pb(48Ca,2n) |
| 253Nog | 1967 | 242Pu(16O,5n),239Pu(18O,4n) |
| 253Nom | 1971 | 249Cf(12C,4n) |
| 254Nog | 1966 | 243Am(15N,4n) |
| 254Nom1 | 1967? | 246Cm(13C,5n),246Cm(12C,4n) |
| 254Nom2 | ~2003 | 208Pb(48Ca,2n) |
| 255No | 1967 | 246Cm(13C,4n),248Cm(12C,5n) |
| 256No | 1967 | 248Cm(12C,4n),248Cm(13C,5n) |
| 257No | 1961? , 1967 | 248Cm(13C,4n) |
| 258No | 1967 | 248Cm(13C,3n) |
| 259No | 1973 | 248Cm(18O,α3n) |
| 260No | ? | 254Es + 22Ne,18O,13C - transfer |
| 261No | unknown | |
| 262No | 1988 | 254Es + 22Ne - transfer (EC of 262Lr) |
Isomerism in nobelium nuclides
254No
The study of K-isomerism was recently studied by physicists at the University of Jyvalkyla (JYFL). They were able to confirm a previously reported K-isomer and detected a second K-isomer. They assigned spins and parities of 8- and 16+ to the two K-isomers.
253No
In 1971, Bemis et al. was able to determine an isomeric level decaying with a half-life of 31 µs from the decay of 257Rf. This was confirmed in 2003 at the GSI by also studying the decay of 257Rf. Further support in the same year from the FLNR appeared with a slightly higher half-life of 43.5 µs, decaying by M2 gamma emission to the ground state.
252No
In a recent study by the GSI into K-isomerism in even-even isotopes, a K-isomer with a half-life of 110 ms was detected for 252No. A spin and parity of 8- was assigned to the isomer.
250No
In 2003, scientists at the FLNR reported that they had been able to synthesise 249No which decayed by SF with a half-life of 54µs. Further work in 2006 by scientists at the ANL showed that the activity was actually due to a K-isomer in 250No. The ground state isomer was also detected with a very short half-life of 3.7µs.
Chemical yields of isotopes
Cold fusion
The table below provides cross-sections and excitation energies for cold fusion reactions producing nobelium isotopes directly. Data in bold represents maxima derived from excitation function measurements. + represents an observed exit channel.
| Projectile | Target | CN | 1n | 2n | 3n | 4n |
|---|---|---|---|---|---|---|
| 48Ca | 208Pb | 256No | 254No: 2050 nb ; 22.3 MeV | |||
| 48Ca | 207Pb | 255No | 253No: 1310 nb ; 22.4 MeV | |||
| 48Ca | 206Pb | 254No | 253No: 58 nb ; 23.6 MeV | 252No: 515 nb ; 23.3 MeV | 251No: 30 nb ; 30.7 MeV | 250No: 260 pb ; 43.9 MeV |
| 48Ca | 204Pb | 252No | 250No:13.2 nb ; 23.2 MeV |
Hot fusion
The table below provides cross-sections and excitation energies for hot fusion reactions producing nobelium isotopes directly. Data in bold represents maxima derived from excitation function measurements. + represents an observed exit channel.
| Projectile | Target | CN | 3n | 4n | 5n | 6n |
|---|---|---|---|---|---|---|
| 26Mg | 232Th | 258No | 254No:1.6 nb | 253No:9 nb | 252No:8 nb | |
| 22Ne | 238U | 260No | 256No:40 nb | 255No:200 nb | 254No:15 nb | |
| 22Ne | 236U | 258No | 254No:7 nb | 253No:25 nb | 252No:15 nb |
Retracted isotopes
249No
In 2003, scientists at the FLNR claimed to have discovered the lightest known isotope of nobelium. However, subsequent work showed that the 54 µs activity was actually due to 250No and the isotope 249No was retracted.
Popular culture
Nobelium was the most recent element "of which the news had come to Harvard" when Tom Lehrer wrote " The Elements Song" and was therefore the element with the highest atomic number to be included.