Molybdenum
2008/9 Schools Wikipedia Selection. Related subjects: Chemical elements
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| General | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Name, Symbol, Number | molybdenum, Mo, 42 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chemical series | transition metals | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Group, Period, Block | 6, 5, d | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Appearance | gray metallic |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Standard atomic weight | 95.94 (2) g·mol−1 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Electron configuration | [Kr] 4d5 5s1 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Electrons per shell | 2, 8, 18, 13, 1 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Physical properties | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Phase | solid | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Density (near r.t.) | 10.28 g·cm−3 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Liquid density at m.p. | 9.33 g·cm−3 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Melting point | 2896 K (2623 ° C, 4753 ° F) |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Boiling point | 4912 K (4639 ° C, 8382 ° F) |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Heat of fusion | 37.48 kJ·mol−1 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Heat of vaporization | 617 kJ·mol−1 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Specific heat capacity | (25 °C) 24.06 J·mol−1·K−1 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Atomic properties | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Crystal structure | cubic body centered | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Oxidation states | 6, 5, 4, 3, 2, 1 (strongly acidic oxide) |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Electronegativity | 2.16 (Pauling scale) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Ionization energies ( more) |
1st: 684.3 kJ·mol−1 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 2nd: 1560 kJ·mol−1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 3rd: 2618 kJ·mol−1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Atomic radius | 145 pm | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Atomic radius (calc.) | 190 pm | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Covalent radius | 145 pm | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Miscellaneous | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Magnetic ordering | no data | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Electrical resistivity | (20 °C) 53.4 n Ω·m | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Thermal conductivity | (300 K) 138 W·m−1·K−1 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Thermal expansion | (25 °C) 4.8 µm·m−1·K−1 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Speed of sound (thin rod) | ( r.t.) 5400 m·s−1 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Young's modulus | 329 GPa | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Shear modulus | 126 GPa | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Bulk modulus | 230 GPa | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Poisson ratio | 0.31 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Mohs hardness | 5.5 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Vickers hardness | 1530 MPa | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Brinell hardness | 1500 MPa | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| CAS registry number | 7439-98-7 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Selected isotopes | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| References | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Molybdenum (pronounced /məˈlɪbdənəm/, from the Greek meaning "lead-like"), is a Group 6 chemical element with the symbol Mo and atomic number 42. It has the sixth-highest melting point of any element, and for this reason it is often used in high-strength steel alloys. Molybdenum is found in trace amounts in plants and animals, although excess molybdenum can be toxic in some animals. Molybdenum was discovered in 1778 by Carl Wilhelm Scheele and first isolated in 1781 by Peter Jacob Hjelm.
Characteristics
Molybdenum is a transition metal with an electronegativity of 1.8 on the Pauling scale and an atomic mass of 95.9 g/mole. It does not react with oxygen or water at room temperature. At elevated temperatures, molybdenum trioxide is formed in the reaction 2Mo + 3O2 → 2MoO3.
In its pure metal form, molybdenum is silvery white and very hard, though it is somewhat more ductile than tungsten. It has a melting point of 2623°C, and only tantalum, osmium, rhenium, and tungsten have higher melting points. Molybdenum burns only at temperatures above 600°C. It also has the lowest heating expansion of any commercially used metal.
Molybdenum has a value of approximately $65,000 per tonne as of 4 May 2007. It maintained a price at or near $10,000 per tonne from 1997 through 2002, and reached a high of $103,000 per tonne in June 2005.
Isotopes
There are 35 known isotopes of molybdenum ranging in atomic mass from 83 to 117, as well as four metastable nuclear isomers. Seven isotopes occur naturally, with atomic masses of 92, 94, 95, 96, 97, 98, and 100. Of these naturally occurring isotopes, five are stable, with atomic masses from 94 to 98. All unstable isotopes of molybdenum decay into isotopes of niobium, technetium, and ruthenium.
Molybdenum-92 and molybdenum-100 are the only naturally occurring isotopes that are not stable. Molybdenum-100 has a half-life of approximately 1×1019 y and undergoes double beta decay into ruthenium-100. Molybdenum-98 is the most common isotope, comprising 24.14% of all molybdenum. Molybdenum isotopes with mass numbers from 111 to 117 all have half-lives of approximately .15 μs.
Occurrence
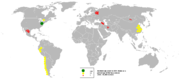
The world's largest producers of molybdenum materials are the United States, Canada, Chile, Russia, and China.
Though molybdenum is found in such minerals as wulfenite (PbMoO4) and powellite (CaMoO4), the main commercial source of molybdenum is molybdenite (MoS2). Molybdenum is mined as a principal ore, and is also recovered as a byproduct of copper and tungsten mining. The large mining areas in Colorado ( Climax) and in British Columbia yield molybdenite while the Chuquicamata mines in northern Chile produce molybdenum as a byproduct of copper mining. The Knaben mine in southern Norway was opened in 1885, making it the first molybdenum mine. It remained open until 1973.
Molybdenum is the 42nd-most-abundant element in the universe, and the 25th-most-abundant element in Earth's oceans, with an average of 10.8 mt/km³. The Russian Luna 24 mission discovered a single molybdenum-bearing grain (1 × 0.6 µm) in a pyroxene fragment taken from Mare Crisium on the Moon.
A side product of molybdenum mining is rhenium. As it is always present in small varying quantities in molybdenite, the only commercial source for rhenium is molybdenum mines.
Compounds
Molybdenum has several common oxidation states, +2 +3 +4 +5 and +6. The highest oxidation state is common in the molybdenum(VI) oxide MoO3 while the normal sulfur compound is molybdenum disulfide MoS2. The broad range of oxidation states shows up in the chlorides of molybdenum:
- Molybdenum(II) chloride MoCl2 (yellow solid),
- Molybdenum(III) chloride MoCl3 (dark red solid),
- Molybdenum(V) chloride MoCl5 (dark green solid),
- Molybdenum(IV) chloride MoCl6 (brown solid),
Like chromium and some other transition metals molybdenum is able to form quadruple bonds
Biological role
The most important use of the molybdenum atom in in living organisms is as a metal hetero-atom at the active site in certain in enzymes. In nitrogen fixation in certain bacteria, the nitrogenase enzyme which is involved in the terminal step of reducing molecular nitrogen, usually contains molybdenum in the active site (though replacement of Mo with iron or vanadium is known).
Though molybdenum forms compounds with various organic molecules, including carbohydrates and amino acids, it is transported throughout the human body as MoO42-. Molybdenum is present in approximately 20 enzymes in animals, including aldehyde oxidase, sulfite oxidase, xanthine oxidase. In some animals, the oxidation of xanthine to uric acid, a process of purine catabolism, is catalyzed by xanthine oxidase, a molybdenum-containing enzyme. The activity of xanthine oxidase is directly proportional to the amount of molybdenum in the body. However, an extremely high concentration of molybdenum reverses the trend, and can act as an inhibitor in both purine catabolism and other processes. Molybdenum concentrations also affect protein synthesis, metabolism, and growth.
In a 70 kg human body, there is approximately 9.3 mg molybdenum, comprising .00001% of the total body mass. It occurs in higher concentrations in the liver and kidneys, and in lower concentrations in the vertebrae. Molybdenum is also present within human tooth enamel and may help prevent the decaying thereof. Pork, lamb, and beef liver each have approximately 1.5 parts molybdenum per million. Other significant dietary sources include green beans, eggs, sunflower seeds, wheat flour, lentils, and cereal grain.
The average daily intake of molybdenum is .3 mg. Daily intake above .4 mg can be toxic. Molybdenum deficiency, caused by less than .05 mg/day, can cause stunted growth, reduced appetite, and impaired reproduction. Sodium tungstate is a competitive inhibitor of molybdenum. Dietary tungsten reduces the concentration of molybdenum in tissues.
Copper-molybdenum antagonism
High amounts of molybdenum can interfere with the body's uptake of copper, both by preventing plasma proteins from binding the copper and by increasing the amount of copper that is excreted in urine. Ruminants that consume high amounts of molybdenum develop symptoms including diarrhea, stunted growth, anaemia, and achromotrichia. These symptoms can be alleviated by the administration of more copper into the system, both in dietary form and by injection. The condition can be aggravated by excess sulfur.
Applications
The ability of molybdenum to withstand extreme temperatures without significantly expanding or softening makes it useful in applications that involve intense heat, including the manufacture of aircraft parts, electrical contacts, industrial motors, and filaments. Molybdenum is also used in alloys for its high corrosion resistance and weldability. Most high-strength steel alloys are .25% to 8% molybdenum. Despite being used in such small portions, more than 43 million kg of molybdenum is used as an alloying agent each year in stainless steels, tool steels, cast irons, and high-temperature superalloys.
Because of its lower density and more stable price, molybdenum is implemented in the place of tungsten. Molybdenum can be implemented both as an alloying agent and as a flame-resistant coating for other metals. Although its melting point is 2623 °C, molybdenum rapidly oxidizes at temperatures above 760 °C, making it better-suited for use in vacuum environments.
Molybdenum disulfide (MoS2) is used as a lubricant and an agent. It forms strong films on metallic surfaces, and is highly resistant to both extreme temperatures and high pressure. Lead molybdate co-precipitated with lead chromate and lead sulfate is a bright-orange pigment used with ceramics and plastics. Molybdenum trioxide (MoO3) is used as an adhesive between enamels and metals. Molybdenum powder is used as a fertilizer for some plants, such as cauliflower.
Also used in NO, NO2, NOx analyzers in power plants for pollution controls. At 350 °C the element acts as a catalyst for NO2/NOx to form only NO molecules for consistent readings by infrared light.
History
Molybdenite (from the Greek Μόλυβδος molybdos, meaning lead), the principal ore from which molybdenum is now extracted, was previously known as molybdena. Molybdena was confused with and often implemented as though it were graphite. Even when the two ores were distinguishable, molybdena was thought to be a lead ore. In 1754, Bengt Qvist examined the mineral and determined that it did not contain lead.
It was not until 1778 that Swedish chemist Carl Wilhelm Scheele realized molybdena was neither graphite nor lead. He and other chemists then correctly assumed that it was the ore of a distinct new element, named molybdenum for the mineral in which it was discovered. Peter Jacob Hjelm successfully isolated molybdenum using carbon and linseed oil in 1781. For a long time there was no industrial use for molybdenum. The French Schneider Electrics company produced the first steel molybdenum alloy armor plates in 1894. Until World War I most other armor factories also used molybdenum alloys. In World War I, some British tanks were protected by 75 mm manganese plating, but this proved to be ineffective. The manganese plates were then replaced with 25 mm molybdenum plating. These allowed for higher speed, greater maneuverability, and, despite being thinner, better protection. The high demand of molybdenum in World War I and World War II and the steep decrease after the wars had a great influence on prices and production of molybdenum.
Precautions
Molybdenum dusts and fumes, as can be generated by mining or metalworking, are not toxic. There are no long-term effects associated with exposure to molybdenum; however, prolonged exposure can cause irritation to the eyes and skin. The direct inhalation or ingestion of molybdenum should also be avoided. OSHA regulations specify the maximum permissible molybdenum exposure in an 8-hour day to be 5 mg/m³. Chronic exposure to 60 to 600 mg Mo/m³ can cause symptoms including fatigue, headaches, and joint pains.