Natural gas
2008/9 Schools Wikipedia Selection. Related subjects: Business; Chemical compounds
Natural gas is a gaseous fossil fuel consisting primarily of methane but including significant quantities of ethane, propane, butane, and pentane—heavier hydrocarbons removed prior to use as a consumer fuel —as well as carbon dioxide, nitrogen, helium and hydrogen sulfide. It is found in oil fields (associated) either dissolved or isolated in natural gas fields (non associated), and in coal beds (as coalbed methane). When methane-rich gases are produced by the anaerobic decay of non-fossil organic material, these are referred to as biogas. Sources of biogas include swamps, marshes, and landfills (see landfill gas), as well as sewage sludge and manure by way of anaerobic digesters, in addition to enteric fermentation particularly in cattle.
Since natural gas is not a pure product, when non associated gas is extracted from a field under supercritical (pressure/temperature) conditions, it may partially condense upon isothermic depressurizing--an effect called retrograde condensation. The liquids thus formed may get trapped by depositing in the pores of the gas reservoir. One method to deal with this problem is to reinject dried gas free of condensate to maintain the underground pressure and to allow reevaporation and extraction of condensates.
Natural gas is often informally referred to as simply gas, especially when compared to other energy sources such as electricity. Before natural gas can be used as a fuel, it must undergo extensive processing to remove almost all materials other than methane. The by-products of that processing include ethane, propane, butanes, pentanes and higher molecular weight hydrocarbons, elemental sulfur, and sometimes helium and nitrogen.
Chemical composition
The primary component of natural gas is methane (CH4), the shortest and lightest hydrocarbon molecule. It often also contains heavier gaseous hydrocarbons such as ethane (C2H6), propane (C3H8) and butane (C4H10), as well as other sulfur containing gases, in varying amounts, see also natural gas condensate. Natural gas that contains hydrocarbons other than methane is called wet natural gas. Natural gas consisting only of methane is called dry natural gas.
| Component | Typical wt. % |
|---|---|
| Methane (CH4) | 70-90 |
| Ethane (C2H6) | 5-15 |
| Propane (C3H8) and Butane (C4H10) | < 5 |
| CO2, N2, H2S, etc. | balance |
Nitrogen, helium, carbon dioxide and trace amounts of hydrogen sulfide, water and odorants can also be present . Natural gas also contains and is the primary market source of helium. Mercury is also present in small amounts in natural gas extracted from some fields. The exact composition of natural gas varies between gas fields.
Organosulfur compounds and hydrogen sulfide are common contaminants which must be removed prior to most uses. Gas with a significant amount of sulfur impurities, such as hydrogen sulfide, is termed sour gas; gas with sulfur or carbon dioxide impurities is acid gas. Processed natural gas that is available to end-users is tasteless and odorless, however, before gas is distributed to end-users, it is odorized by adding small amounts of odorants (mixtures of t-butyl mercaptan, isopropyl mercaptanthiol, tetrahydrothiophene, dimethyl sulfide and other sulfur compounds), to assist in leak detection. Processed natural gas is, in itself, harmless to the human body, however, natural gas is a simple asphyxiant and can kill if it displaces air to the point where the oxygen content will not support life.
Natural gas can also be hazardous to life and property through an explosion. Natural gas is lighter than air, and so tends to escape into the atmosphere. But when natural gas is confined, such as within a house, gas concentrations can reach explosive mixtures and, if ignited, result in blasts that could destroy buildings. Methane has a lower explosive limit of 5% in air, and an upper explosive limit of 15%. Explosive concerns with compressed natural gas used in vehicles are almost non-existent, due to the escaping nature of the gas, and the need to maintain concentrations between 5% and 15% to trigger explosions.
Energy content, statistics and pricing
Quantities of natural gas are measured in normal cubic meters (corresponding to 0°C at 101.325 kPaA) or in standard cubic feet (corresponding to 60 °F (16 °C) and 14.73 PSIA). The gross heat of combustion of one normal cubic meter of commercial quality natural gas is around 39 megajoules (≈10.8 kWh), but this can vary by several percent. In US units, one standard cubic foot of natural gas produces around 1,030 British Thermal Units (BTUs). The actual heating value when the water formed does not condense is the net heat of combustion and can be as much as 10% less.
The price of natural gas varies greatly depending on location and type of consumer. In 2007, a price of $7 per 1,000 cubic feet (28 m³) was typical in the United States. The typical caloric value of natural gas is roughly 1,000 BTU per cubic foot, depending on gas composition. This corresponds to around $7 per million BTU's, or around $7 per gigajoule. In April 2008, the wholesale price was $10 per 1,000 cubic feet (28 m³) ($10/MBTU) . The residential price varies from 50% to 300% more than the wholesale price. At the end of 2007, this was $12-$16 per 1000 ft3 (or MBTU) . Natural gas in the United States is traded as a futures contract on the New York Mercantile Exchange. Each contract is for 10,000 MMBTU ( gigajoules), or 10 billion BTU's. Thus, if the price of gas is $10 per million BTU's on the NYMEX, the contract is worth $100,000.
In the United States, retail sales are often in units of therms (th); 1 therm = 100,000 BTU. Gas meters measure the volume of gas used, and this is converted to therms by multiplying the volume by the energy content of the gas used during that period, which varies slightly over time. Wholesale transactions are generally done in decatherms (Dth), or in thousand decatherms (MDth), or in million decatherms (MMDth). A million decatherms is roughly a billion cubic feet of natural gas.
Natural gas is also traded as a commodity in Europe, principally at the United Kingdom NBP and related European hubs, such as the TTF in the Netherlands.
In the rest of the world, LNG ( liquified natural gas) and LPG ( liquified petroleum gas) is traded in metric tons or mmBTU as spot deliveries. Long term contracts are signed in metric tons. The LNG and LPG is transported by specialized transport ships, as the gas is liquified at cryogenic temperatures. The specification of each LNG/LPG cargo will usually contain the energy content, but this information is in general not available to the public.
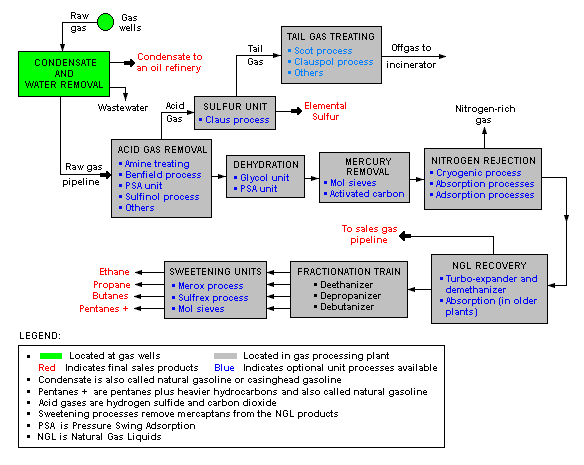
Natural gas processing
The image below is a schematic block flow diagram of a typical natural gas processing plant. It shows the various unit processes used to convert raw natural gas into sales gas pipelined to the end user markets.
The block flow diagram also shows how processing of the raw natural gas yields byproduct sulfur, byproduct ethane, and natural gas liquids (NGL) propane, butanes and natural gasoline (denoted as pentanes +).
Storage and transport
The major difficulty in the use of natural gas is transportation and storage because of its low density. Natural gas pipelines are economical, but are impractical across oceans. Many existing pipelines in North America are close to reaching their capacity, prompting some politicians representing colder areas to speak publicly of potential shortages.
LNG carriers can be used to transport liquefied natural gas (LNG) across oceans, while tank trucks can carry liquefied or compressed natural gas (CNG) over shorter distances. They may transport natural gas directly to end-users, or to distribution points such as pipelines for further transport. These may have a higher cost, requiring additional facilities for liquefaction or compression at the production point, and then gasification or decompression at end-use facilities or into a pipeline.
In the past, the natural gas which was recovered in the course of recovering petroleum could not be profitably sold, and was simply burned at the oil field (known as flaring). This wasteful practice is now illegal in many countries. Additionally, companies now recognize that value for the gas may be achieved with LNG, CNG, or other transportation methods to end-users in the future. The gas is now re- injected back into the formation for later recovery. This also assists oil pumping by keeping underground pressures higher. In Saudi Arabia, in the late 1970s, a "Master Gas System" was created, ending the need for flaring. Satellite observation unfortunately shows that some large gas-producing countries still use flaring and venting routinely. The natural gas is used to generate electricity and heat for desalination. Similarly, some landfills that also discharge methane gases have been set up to capture the methane and generate electricity.
Natural gas is often stored in underground caverns formed inside depleted gas reservoirs from previous gas wells, salt domes, or in tanks as liquefied natural gas. The gas is injected during periods of low demand and extracted during periods of higher demand. Storage near the ultimate end-users helps to best meet volatile demands, but this may not always be practicable.
With 15 nations accounting for 84% of the world-wide production, access to natural gas has become a significant factor in international economics and politics. In this respect, control over the pipelines is a major strategic factor.
Use
Power generation
Natural gas is a major source of electricity generation through the use of gas turbines and steam turbines. Particularly high efficiencies can be achieved through combining gas turbines with a steam turbine in combined cycle mode. Natural gas burns cleaner than other fossil fuels, such as oil and coal, and produces less carbon dioxide per unit energy released. For an equivalent amount of heat, burning natural gas produces about 30% less carbon dioxide than burning petroleum and about 45% less than burning coal. Combined cycle power generation using natural gas is thus the cleanest source of power available using fossil fuels, and this technology is widely used wherever gas can be obtained at a reasonable cost. Fuel cell technology may eventually provide cleaner options for converting natural gas into electricity, but as yet it is not price-competitive.
Hydrogen
Natural gas can be used to produce hydrogen, with one common method being the hydrogen reformer. Hydrogen has various applications: it is a primary feedstock for the chemical industry, a hydrogenating agent, an important commodity for oil refineries, and a fuel source in hydrogen vehicles.
Natural Gas Vehicles
Compressed natural gas (methane) is a cleaner alternative to other automobile fuels such as gasoline (petrol) and diesel. As of 2005, the countries with the largest number of natural gas vehicles were Argentina, Brazil, Pakistan, Italy, Iran, and the USA. The energy efficiency is generally equal to that of gasoline engines, but lower compared with modern diesel engines. Benzene (aka gasoline, petrol) vehicles converted to run on Natural Gas suffer because of the low compression ratio of their engines, resulting in a cropping of delivered power while running on natural gas (10%-15%). CNG-specific engines, however, use a higher compression ratio due to this fuel's higher octane number of 120-130.
Residential domestic use
Natural gas is supplied to homes, where it is used for such purposes as cooking in natural gas-powered ranges and/or ovens, natural gas-heated clothes dryers, heating/ cooling and central heating. Home or other building heating may include boilers, furnaces, and water heaters. CNG is used in rural homes without connections to piped-in public utility services, or with portable grills. However, due to CNG being less economical than LPG, LPG (Propane) is the dominant source of rural gas.
Fertilizer
Natural gas is a major feedstock for the production of ammonia, via the Haber process, for use in fertilizer production.
Aviation
Russian aircraft manufacturer Tupolev is currently running a development program to produce LNG- and hydrogen-powered aircraft. The program has been running since the mid-1970s, and seeks to develop LNG and hydrogen variants of the Tu-204 and Tu-334 passenger aircraft, and also the Tu-330 cargo aircraft. It claims that at current market prices, an LNG-powered aircraft would cost 5,000 roubles less to operate per ton, roughly equivalent to 60%, with considerable reductions to carbon monoxide, hydrocarbon and nitrogen oxide emissions.
The advantages of liquid methane as a jet engine fuel are that it has more specific energy than the standard kerosene mixes and that its low temperature can help cool the air which the engine compresses for greater volumetric efficiency, in effect replacing an intercooler. Alternatively, it can be used to lower the temperature of the exhaust.
Other
Natural gas is also used in the manufacture of fabrics, glass, steel, plastics, paint, and other products.
Safety
In any form, a minute amount of odorant such as t-butyl mercaptan, with a rotting-cabbage-like smell, is added to the otherwise colorless and almost odorless gas, so that leaks can be detected before a fire or explosion occurs. Sometimes a related compound, thiophane is used, with a rotten-egg smell. Adding odorant to natural gas began in the United States after the 1937 New London School explosion. The buildup of gas in the school went unnoticed, killing three hundred students and faculty when it ignited. Odorants are considered non-toxic in the extremely low concentrations occurring in natural gas delivered to the end user.
In mines, where methane seeping from rock formations has no odour, sensors are used, and mining apparatuses have been specifically developed to avoid ignition sources, e.g., the Davy lamp.
Explosions caused by natural gas leaks occur a few times each year. Individual homes, small businesses and boats are most frequently affected when an internal leak builds up gas inside the structure. Frequently, the blast will be enough to significantly damage a building but leave it standing. In these cases, the people inside tend to have minor to moderate injuries. Occasionally, the gas can collect in high enough quantities to cause a deadly explosion, disintegrating one or more buildings in the process. The gas usually dissipates readily outdoors, but can sometimes collect in dangerous quantities if weather conditions are right. However, considering the tens of millions of structures that use the fuel, the individual risk of using natural gas is very low.
Some gas fields yield sour gas containing hydrogen sulfide (H2S). This untreated gas is toxic. Amine gas treating, an industrial scale process which removes acidic gaseous components, is often used to remove hydrogen sulfide from natural gas.
Extraction of natural gas (or oil) leads to decrease in pressure in the reservoir. This in turn may lead to subsidence at ground level. Subsidence may affect ecosystems, waterways, sewer and water supply systems, foundations, etc.
Natural Gas heating systems are the leading cause of carbon monoxide deaths in the United States, according to the US Consumer Product Safety Commission. When a natural gas heating system malfunctions, it produces odorless carbon monoxide. With no fumes or smoke to give warning, poisoning victims are easily asphyxiated by the carbon monoxide. Detectors are available that warn of carbon monoxide and/or explosive gas (methane, propane, etc.)
Cost comparison with heating oil in the USA
It is difficult to evaluate the cost of heating a home with natural gas compared to that of heating oil, because of differences of energy conversion efficiency, and the widely fluctuating price of crude oil. However, for illustration, one can calculate a representative cost per BTU. Assuming the following current values:
-
- For natural gas
- One cubic foot of natural gas produces about 1,030 BTU (38.4 MJ/m³)
- The price of natural gas is $9.00 per thousand cubic feet ($0.32/m³)
-
- For heating oil
- One US gallon of heating oil produces about 138,500 BTU (38.6 MJ/l)
- The price of heating oil is $2.50 per US gallon ($0.66/l)
This gives a cost of $8.70 per million BTU ($8.30/GJ) for natural gas, as compared to $18 per million BTU ($17/GJ) for fuel oil. Of course, such comparisons fluctuate with time and vary from place to place dependent on the cost of the raw materials and local taxation.