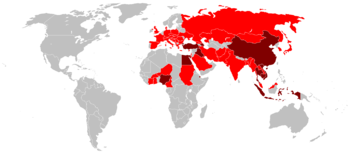
Global spread of H5N1
2008/9 Schools Wikipedia Selection. Related subjects: Environment
| Highly pathogenic H5N1 | |
|---|---|
| Countries with poultry or wild birds killed by highly pathogenic H5N1. | |
| Countries with human cases of highly pathogenic H5N1. | |
The global spread of highly pathogenic H5N1 in birds is considered a significant pandemic threat.
While other H5N1 strains are known, they are significantly different from a current, highly pathogenic H5N1 strain on a genetic level, making the global spread of this new strain unprecedented. The H5N1 strain is a fast-mutating, highly pathogenic avian influenza virus (HPAI) found in multiple bird species. It is both epizootic (an epidemic in non-humans) and panzootic (a disease affecting animals of many species especially over a wide area). Unless otherwise indicated, "H5N1" in this article refers to the recent highly pathogenic strain of H5N1.
"Since 1997, studies of H5N1 indicate that these viruses continue to evolve, with changes in antigenicity and internal gene constellations; an expanded host range in avian species and the ability to infect felids; enhanced pathogenicity in experimentally infected mice and ferrets, in which they cause systemic infections; and increased environmental stability."
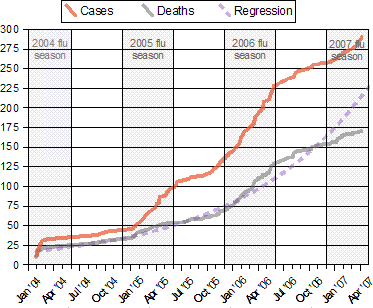 |
Notes:
- Source WHO Confirmed Human Cases of H5N1
- "[T]he incidence of human cases peaked, in each of the three years in which cases have occurred, during the period roughly corresponding to winter and spring in the northern hemisphere. If this pattern continues, an upsurge in cases could be anticipated starting in late 2006 or early 2007." Avian influenza – epidemiology of human H5N1 cases reported to WHO
- The regression curve for deaths is y = a + ek x, and is shown extended through the end of April, 2007.
Tens of millions of birds have died of H5N1 influenza and hundreds of millions of birds have been slaughtered and disposed of, to limit the spread of H5N1. Countries that have reported one or more major highly pathogenic H5N1 outbreaks in birds (causing at least thousands but in some cases millions of dead birds) are (in order of first outbreak occurrence): Korea, Vietnam, Japan, Thailand, Cambodia, Laos, Indonesia, China, Malaysia, Russia, Kazakhstan, Mongolia, Turkey, Romania, Croatia, Ukraine, Cyprus, Iraq, Nigeria, Egypt, India, France, Niger, Bosnia, Azerbaijan, Albania, Cameroon, Myanmar, Afghanistan, Israel, Pakistan, Jordan, Burkina Faso, Germany, Sudan, Ivory Coast, Djibouti, Hungary, United Kingdom, Kuwait, Bangladesh, Saudi Arabia, Ghana, Czech Republic, Togo.
Highly pathogenic H5N1 has been found in birds in the wild in numerous other countries: Austria, Bulgaria, Denmark, Greece, Iran, Italy, Poland, Serbia and Montenegro, Slovakia, Slovenia, Spain, Sweden, Switzerland. Surveillance of H5N1 in humans, poultry, wild birds, cats and other animals remains very weak in many parts of Asia and Africa. Much remains unknown about the exact extent of its spread.
H5N1 has low pathogenic varieties endemic in birds in North America. H5N1 has a highly pathogenic variety that is endemic in dozens of species of birds throughout south Asia and is threatening to become endemic in birds in west Asia and Africa. So far, it is very difficult for humans to become infected with H5N1. The presence of highly pathogenic (deadly) H5N1 around the world in both birds in the wild (swans, magpies, ducks, geese, pigeons, eagles, etc.) and in chickens and turkeys on farms has been demonstrated in millions of cases with the virus isolate actually sequenced in hundreds of cases yielding definitive proof of the evolution of this strain of this subtype of the species Influenzavirus A (bird flu virus).
|
According to Robert Webster:
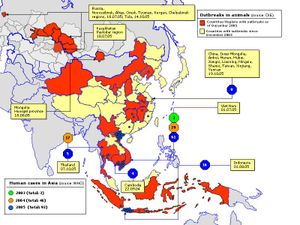
- "The epicenters of both the Asian influenza pandemic of 1957 and the Hong Kong influenza pandemic of 1968 were in Southeast Asia, and it is in this region that multiple clades of H5N1 influenza virus have already emerged. The Asian H5N1 virus was first detected in Guangdong Province, China, in 1996, when it killed some geese, but it received little attention until it spread through live-poultry markets in Hong Kong to humans in May 1997, killing 6 of 18 infected persons. [...] From 1997 to May 2005, H5N1 viruses were largely confined to Southeast Asia, but after they had infected wild birds in Qinghai Lake, China, they rapidly spread westward. [...] The intermittent spread to humans will continue, and the virus will continue to evolve." Map
Human and bird cases
| Country | Report dates |
Total |
|||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2003 | 2004 | 2005 | 2006 | 2007 | 2008 | ||||||||||||||||
| cases | deaths | cases | deaths | cases | deaths | cases | deaths | cases | deaths | cases | deaths | cases | deaths | ||||||||
| 8 | 5 | 63% | 8 | 5 | 63% | ||||||||||||||||
| 1 | 0 | 0% | 1 | 0 | 0% | ||||||||||||||||
| 4 | 4 | 100% | 2 | 2 | 100% | 1 | 1 | 100% | 7 | 7 | 100% | ||||||||||
| 1 | 1 | 100% | 8 | 5 | 63% | 13 | 8 | 62% | 5 | 3 | 60% | 3 | 3 | 100% | 30 | 20 | 67% | ||||
| 1 | 0 | 0% | 1 | 0 | 0% | ||||||||||||||||
| 18 | 10 | 56% | 25 | 9 | 36% | 7 | 3 | 43% | 50 | 22 | 44% | ||||||||||
| 20 | 13 | 65% | 55 | 45 | 82% | 42 | 37 | 88% | 18 | 15 | 83% | 135 | 110 | 81% | |||||||
| 3 | 2 | 67% | 3 | 2 | 67% | ||||||||||||||||
| 2 | 2 | 100% | 2 | 2 | 100% | ||||||||||||||||
| 1 | 0 | 0% | 1 | 0 | 0% | ||||||||||||||||
| 1 | 1 | 100% | 1 | 1 | 100% | ||||||||||||||||
| 3 | 1 | 33% | 3 | 1 | 33% | ||||||||||||||||
| 17 | 12 | 71% | 5 | 2 | 40% | 3 | 3 | 100% | 25 | 17 | 68% | ||||||||||
| 12 | 4 | 33% | 12 | 4 | 33% | ||||||||||||||||
| 3 | 3 | 100% | 29 | 20 | 69% | 61 | 19 | 31% | 8 | 5 | 63% | 5 | 5 | 100% | 106 | 52 | 49% | ||||
| Total | 4 | 4 | 100% | 46 | 32 | 70% | 98 | 43 | 44% | 115 | 79 | 69% | 88 | 59 | 67% | 34 | 26 | 76% | 385 | 243 | 63% |
| Source: World Health Organization Communicable Disease Surveillance & Response (CSR) | |||||||||||||||||||||
1959-1997
- A highly pathogenic strain of H5N1 caused flu outbreaks with significant spread to numerous farms, resulting in great economic losses in 1959 in Scotland in chickens and in 1991 in England in turkeys. These strains were somewhat similar to the current pathogenic strain of H5N1 in two of its ten genes, the gene that causes it to be type H5 and the gene that causes it to be N1. The other genes can and have been reassorted from other subtypes of the bird flu species (their ease at exchanging genes is part of what makes them all one species). Evolution by reassortment of H5N1 from 1999 to 2002 created the Z genotype which became the dominant strain of highly pathogenic H5N1 in 2004 and is now spreading across the entire world in both wild and domestic birds.
- "The precursor of the H5N1 influenza virus that spread to humans in 1997 was first detected in Guangdong, China, in 1996, when it caused a moderate number of deaths in geese and attracted very little attention."
- In 1997, in Hong Kong, 18 humans were infected and 6 died in the first known case of H5N1 infecting humans.
- On 28 December to 29 December 1997, 1.3 million of chickens were killed by Hong Kong Government. The government also suspended the import of chickens from mainland China.
2003
- "Human disease associated with influenza A subtype H5N1 re-emerged in January 2003, for the first time since an outbreak in Hong Kong in 1997." Three people in one family were infected after visiting Fujian province in mainland China and 2 died.
- By midyear of 2003 outbreaks of poultry disease caused by H5N1 occurred in Asia, but were not recognized as such. That December animals in a Thai zoo died after eating infected chicken carcasses. Later that month H5N1 infection was detected in 3 flocks in the Republic of Korea.
- H5N1 in China in this and later periods is less than fully reported. Blogs have described many discrepancies between official China government announcements concerning H5N1 and what people in China see with their own eyes. Many reports of total H5N1 cases exclude China due to widespread disbelief in China's official numbers.
2004
In January 2004 a major new outbreak of H5N1 surfaced in Vietnam and Thailand's poultry industry, and within weeks spread to ten countries and regions in Asia, including Indonesia, South Korea, Japan and China. In October 2004 researchers discovered H5N1 is far more dangerous than previously believed because waterfowl, especially ducks, were directly spreading the highly pathogenic strain of H5N1 to chickens, crows, pigeons, and other birds and that it was increasing its ability to infect mammals as well. From this point on, avian influenza experts increasingly refer to containment as a strategy that can delay but not prevent a future avian flu pandemic.
2005
In January 2005 an outbreak of avian influenza affected thirty three out of sixty four cities and provinces in Vietnam, leading to the forced killing of nearly 1.2 million poultry. Up to 140 million birds are believed to have died or been killed because of the outbreak. In April 2005 there begins an unprecedented die-off of over 6,000 migratory birds at Qinghai Lake in central China over three months. This strain of H5N1 is the same strain as is spread west by migratory birds over at least the next ten months. In August 2005 H5N1 spread to Kazakhstan, Mongolia and Russia. On September 29, 2005, David Nabarro, the newly appointed Senior United Nations System Coordinator for Avian and Human Influenza, warned the world that an outbreak of avian influenza could kill 5 to 150 million people. David Nabarro later stated that as the virus had spread to migratory birds, an outbreak could start in Africa or the Middle East. Later in 2005 H5N1 spread to Turkey, Romania, Croatia and Kuwait.
2006
- In the first two months of 2006 H5N1 spread to other Asian countries (such as India), north Africa, and Europe in wild bird populations possibly signaling the beginning of H5N1 being endemic in wild migratory bird populations on multiple continents for decades, permanently changing the way poultry are farmed. In addition, the spread of highly pathogenic H5N1 to wild birds, birds in zoos and even sometimes to mammals (example: pet cats) raises many unanswered questions concerning best practices for threat mitigation, trying to balance reducing risks of human and nonhuman deaths from the current nonpandemic strain with reducing possible pandemic deaths by limiting its chances of mutating into a pandemic strain. Not using vaccines can result in the need to kill significant numbers of farm and zoo birds, while using vaccines can increase the chance of a flu pandemic.
- By April 2006 scientists had concluded that containment had failed due to the role of wild birds in transmitting the virus and were now emphasizing far more comprehensive risk mitigation and management measures.
- In June 2006 WHO predicted an upsurge in human deaths due to H5N1 during late 2006 or early 2007 following a summer/fall lull in most countries, as H5N1 appears to be somewhat seasonal in nature. In July and August 2006 significantly increased numbers of bird deaths due to H5N1 were recorded in Cambodia, China, Laos, Nigeria, and Thailand while continuing unabated a rate unparalleled in Indonesia.
- In September, Egypt and Sudan joined the list of nations seeing a resurgence of bird deaths due to H5N1.
- In November and December, South Korea and Vietnam joined the list of nations seeing a resurgence of bird deaths due to H5N1.
2007
In January, Japan, Hungary, Russia, and the United Kingdom joined the list of nations seeing a resurgence of bird deaths due to H5N1. In February, Pakistan, Turkey, Afghanistan, and Myanmar joined the list and Kuwait saw its first major outbreak of H5N1 avian influenza.
In March Bangladesh and Saudi Arabia each saw their first major outbreak of H5N1 avian influenza and Ghana in May.
As H5N1 continued killing many birds and a few people throughout the spring in countries where it is now endemic, in June Malaysia and Germany saw a resurgence of bird deaths due to H5N1, while the Czech Republic and Togo experienced their first major outbreak of H5N1 avian influenza.
In July France and India also saw a resurgence of bird deaths due to H5N1.
2008
January
- January 24, 2008: China's health ministry has confirmed a 22-year-old man has died from H5N1 in central Hunan province.
February
- February 26, 2008: H5N1 killed a school teacher from northern Vietnam in the country's 51st death from the disease, and health officials fretted that the virus would spread further.
- February 28, 2008: There are no indications that H5N1 is becoming a bigger problem in China despite the deaths of three people from the disease this year, the World Health Organisation said Wednesday.
March
- March 4, 2008: H5N1 virus confirmed as the cause of death for a 25 year old female from Sennoris District, Fayum Governorate, Egypt.
June
- June 7, 2008: Hong Kong found the H5N1 bird flu virus at a poultry stall in Sham Shui Po. 2,700 birds were ordered to be killed by the local government. A new regulation require all live chickens not sold by 8pm to be killed. The chairman of the Hong Kong Poultry Wholesalers Association said the govenrment's decision makes it very difficult for their business to continue. Retailers who keep live poultry after 8pm is now subject to a fine of HK$50,000 and six months imprisonment.
Pig cases
Avian influenza virus H3N2 is endemic in pigs (" swine flu") in China and has been detected in pigs in Vietnam, increasing fears of the emergence of new variant strains. Health experts say pigs can carry human influenza viruses, which can combine (i.e. exchange homologous genome sub-units by genetic reassortment) with H5N1, passing genes and mutating into a form which can pass easily among humans. H3N2 evolved from H2N2 by antigenic shift and caused the Hong Kong Flu pandemic of 1968 and 1969 that killed up to 750,000 humans. The dominant strain of annual flu in humans in January 2006 is H3N2. Measured resistance to the standard antiviral drugs amantadine and rimantadine in H3N2 in humans has increased to 91% in 2005. A combination of these two subtypes of the species known as the avian influenza virus in a country like China is a worst case scenario. In August 2004, researchers in China found H5N1 in pigs.
In 2005 it was discovered that H5N1 "could be infecting up to half of the pig population in some areas of Indonesia, but without causing symptoms [...] Chairul Nidom, a virologist at Airlangga University's tropical disease centre in Surabaya, Java, was conducting independent research earlier this year. He tested the blood of 10 apparently healthy pigs housed near poultry farms in western Java where avian flu had broken out, Nature reported. Five of the pig samples contained the H5N1 virus. The Indonesian government has since found similar results in the same region, Nature reported. Additional tests of 150 pigs outside the area were negative."
Felidae (cats)
"In Bangkok, Thailand, all the cats in one household are known to have died of H5N1 in 2004. Tigers and leopards in Thai zoos also died, while last year two cats near an outbreak in poultry and people in Iraq were confirmed to have died of H5N1, as were three German cats that ate wild birds. In Austria cats were infected but remained healthy". Cats in Indonesia were also found to have been infected with H5N1.
The spread to more and more types and populations of birds and the ability of felidae (cats) to catch H5N1 from eating this natural prey means the creation of a reservoir for H5N1 in cats where the virus can adapt to mammals is one of the many possible pathways to a pandemic.
October 2004
Variants have been found in a number of domestic cats, leopards and tigers in Thailand, with high lethality. "The Thailand Zoo tiger outbreak killed more than 140 tigers, causing health officials to make the decision to cull all the sick tigers in an effort to stop the zoo from becoming a reservoir for H5N1 influenza. A study of domestic cats showed H5N1 virus infection by ingestion of infected poultry and also by contact with other infected cats (Kuiken et al., 2004)." The initial OIE report reads: "the clinical manifestations began on 11 October 2004 with weakness, lethargy, respiratory distress and high fever (about 41-42 degrees Celsius). There was no response to any antibiotic treatment. Death occurred within three days following the onset of clinical signs with severe pulmonary lesions."
February 28, 2006
A dead cat infected with the H5N1 bird flu virus was found in Germany.
March 6, 2006
Hans Seitinger, the top agriculture official in the southern state of Styria, Austria announced that several still living cats in Styria have tested positive for H5N1:
August 2006
It was announced in the August 2006 CDC EID journal that while literature describing HPAI H5N1 infection in cats had been limited to a subset of clade I viruses; a Qinghai-like virus (they are genetically distinct from other clade II viruses) killed up to five cats and 51 chickens from February 3 to February 5, 2006 in Grd Jotyar (~10 km north of Erbil City, Iraq). Two of the cats were available for examination.
- "An influenza A H5 virus was present in multiple organs in all species from the outbreak site in Grd Jotyar (Table). cDNA for sequencing was amplified directly from RNA extracts from pathologic materials without virus isolation. On the basis of sequence analysis of the full HA1 gene and 219 amino acids of the HA2 gene, the viruses from the goose and 1 cat from Grd Jotyar and from the person who died from Sarcapcarn (sequence derived from PCR amplification from first-passage egg material) are >99% identical at the nucleotide and amino acid levels (GenBank nos. DQ435200–02). Thus, no indication of virus adaptation to cats was found. The viruses from Iraq are most closely related to currently circulating Qinghai-like viruses, but when compared with A/bar-headed goose/Qinghai/65/2005 (H5N1) (GenBank no. DQ095622), they share only 97.4% identity at the nucleic acid level with 3 amino acid substitutions of unknown significance. On the other hand, the virus from the cat is only 93.4% identical to A/tiger/Thailand/CU-T4/2004(H5N1) (GenBank no. AY972539). These results are not surprising, given that these strains are representative of different clades (8,9). Sequencing of 1,349 bp of the N gene from cat 1 and the goose (to be submitted to GenBank) show identity at the amino acid level, and that the N genes of viruses infecting the cat and goose are >99% identical to that of A/bar-headed goose/Qinghai/65/2005(H5N1). These findings support the notion that cats may be broadly susceptible to circulating H5N1 viruses and thus may play a role in reassortment, antigenic drift, and transmission."
January 24, 2007
"Chairul Anwar Nidom of Airlangga University in Surabaya, Indonesia, told journalists last week that he had taken blood samples from 500 stray cats near poultry markets in four areas of Java, including the capital, Jakarta, and one area in Sumatra, all of which have recently had outbreaks of H5N1 in poultry and people. Of these cats, 20% carried antibodies to H5N1. This does not mean that they were still carrying the virus, only that they had been infected - probably through eating birds that had H5N1. Many other cats that were infected are likely to have died from the resulting illness, so many more than 20% of the original cat populations may have acquired H5N1."
Mammals in general
H5N1 has been transmitted in laboratories to many species including mice and ferrets to study its effects.
H5N1 was transmitted in the wild to three civet cats in Vietnam in August 2005 and a stone marten in Germany in March 2006.
The BBC reported that a stray dog in Azerbaijan died from the disease on March 15, 2006.
Experts believe more work is needed to determine the role of mammals in the epidemiology of H5N1. Officials are not doing enough to monitor cats, dogs and other carnivores for their possible role in transmitting H5N1. People living in areas where the A(H5N1) virus has infected birds are advised to keep their cats indoors. "Cats can be infected through the respiratory tract. Cats can also be infected when they ingest the virus, which is a novel route for influenza transmission in mammals. But cats excrete only one-thousandth the amount of virus that chickens do [...] The concern is that if large numbers of felines and other carnivores become infected, the virus might mutate in a series of events that could lead to an epidemic among humans. Dogs, foxes, seals and other carnivores may be vulnerable to A(H5N1) virus infection, Dr. Osterhaus said. Tests in Thailand have shown that the virus has infected dogs without causing apparent symptoms."