Chemical synapse
2008/9 Schools Wikipedia Selection. Related subjects: Biology
Chemical synapses are specialized junctions through which neurons signal to each other and to non-neuronal cells such as those in muscles or glands. Chemical synapses allow neurons to form interconnected circuits within the central nervous system. They are thus crucial to the biological computations that underlie perception and thought. They provide the means through which the nervous system connects to and controls the other systems of the body, for example the specialized synapse between a motor neuron and a muscle cell is called a neuromuscular junction.
Young children have about 1016 synapses (10 quadrillion). This number declines with age, stabilizing by adulthood. Estimates for adults vary from 1015 to 5 × 1015 (1-5 quadrillion) synapses.
The word "synapse" comes from "synaptein", which Sir Charles Scott Sherrington and colleagues coined from the Greek "syn-" ("together") and "haptein" ("to clasp"). Chemical synapses are not the only type of biological synapse: electrical and immunological synapses exist as well. Without a qualifier, however, "synapse" commonly refers to a chemical synapse.
Structure
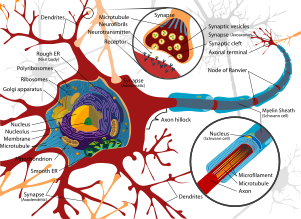
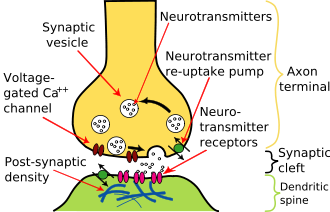
Chemical synapses pass information directionally from a presynaptic cell to a postsynaptic cell and are therefore asymmetric in structure and function. The presynaptic terminal, or synaptic bouton, is a specialized area within the axon of the presynaptic cell that contains neurotransmitters enclosed in small membrane bound spheres called synaptic vesicles. Synaptic vesicles are docked at the presynaptic plasma membrane at regions called active zones (AZ).
Immediately opposite is a region of the postsynaptic cell containing neurotransmitter receptors; for synapses between two neurons the postsynaptic region may be found on the dendrites or cell body. Immediately behind the postsynaptic membrane is an elaborate complex of interlinked proteins called the postsynaptic density (PSD).
Proteins in the PSD are involved in anchoring and trafficking neurotransmitter receptors and modulating the activity of these receptors. The receptors and PSDs are often found in specialized protrusions from the main dendritic shaft called dendritic spines.
Between the pre- and postsynaptic cells is a gap about 20nm wide called the synaptic cleft. The small volume of the cleft allows neurotransmitter concentration to be raised and lowered rapidly. The membranes of the two adjacent cells are held together by cell adhesion proteins.
Signaling across chemical synapses
Neurotransmitter release
The release of a neurotransmitter is triggered by the arrival of a nerve impulse (or action potential) and occurs through an unusually rapid process of cellular secretion, also known as exocytosis: Within the presynaptic nerve terminal, vesicles containing neurotransmitter sit "docked" and ready at the synaptic membrane. The arriving action potential produces an influx of calcium ions through voltage-dependent, calcium-selective ion channels at the down stroke of the action potential (tail current). Calcium ions then trigger a biochemical cascade which results in vesicles fusing with the presynaptic membrane and releasing their contents to the synaptic cleft within 180 µsec of calcium entry. Vesicle fusion is driven by the action of a set of proteins in the presynaptic terminal known as SNAREs.
The membrane added by this fusion is later retrieved by endocytosis and recycled for the formation of fresh neurotransmitter-filled vesicles.
Receptor binding
Receptors on the opposite side of the synaptic gap bind neurotransmitter molecules and respond by opening nearby ion channels in the postsynaptic cell membrane, causing ions to rush in or out and changing the local transmembrane potential of the cell. The resulting change in voltage is called a postsynaptic potential. In general, the result is excitatory, in the case of depolarizing currents, or inhibitory in the case of hyperpolarizing currents. Whether a synapse is excitatory or inhibitory depends on what type(s) of ion channel conduct the postsynaptic current display(s), which in turn is a function of the type of receptors and neurotransmitter employed at the synapse.
Termination
The signal is terminated by either breakdown of neurotransmitters, or reuptake, the latter is mainly in the presynaptic neuron to avail recycling of the transmitter.
Reuptake
Following fusion of the synaptic vesicles and release of transmitter molecules into the synaptic cleft, small neurotransmitters, such as glycine, are rapidly cleared from the space for recycling by specialized membrane proteins in the presynaptic or postsynaptic membrane.
Breakdown
Some neurotransmitters, e.g. acetylcholine and large ones such as peptides, are broken down without any direct reuptake. The choline part of acetylcholine, however, is to a large degree taken up by the presynaptic neuron for recycling. Peptides, on the other hand, must be resynthesized from the neuron soma.
Modulation of synaptic transmission
Synaptic transmission can be modulated by e.g. desensitization, homotropic and heterotropic modulation:
Desensitization
Desensitization of the postsynaptic receptors is a decrease in response to the same neurotransmitter stimulus. It means that the strength of a synapse may in effect diminish as a train of action potentials arrive in rapid succession--a phenomenon that gives rise to the so-called frequency dependence of synapses. The nervous system exploits this property for computational purposes, and can tune its synapses through such means as phosphorylation of the proteins involved.
Homotropic modulation
Homotropic modulation is a modulation of the presynaptic neuron by its own neurotransmitters, i.e. a form of autocrine signaling. The modulation can include size, number and replenishment rate of vesicles. It is often inhibitory, with the effect of presynaptic inhibition, making the neurotransmitter self-regulating.
One example are neurons of the sympathetic nervous system (SNS), which release noradrenaline, which, besides from affecting postsynaptic receptors, also affect α2-adrenergic receptors, inhibiting further release of noradrenaline. This effect is utilized with clonidine to perform inhibitory effects on the SNS.
Heterotropic modulation
Heterotropic modulation is a modulation of presynaptic terminals of nearby neurons. Again, the modulation can include size, number and replenishment rate of vesicles.
One example are again neurons of the sympathetic nervous system, which release noradrenaline, which, in addition, generate inhibitory effect on presynaptic terminals of neurons of the parasympathetic nervous system.
Pharmacological intervention
For example, a class of drugs known as selective serotonin reuptake inhibitors or SSRIs affect certain synapses by inhibiting the reuptake of the neurotransmitter serotonin. In contrast, one important excitatory neurotransmitter, acetylcholine, is first broken down into acetate and choline by the enzyme acetylcholinesterase prior to removal from the synapse.
Integration of synaptic inputs
In general, if an excitatory synapse is strong, an action potential in the presynaptic neuron will trigger another in the postsynaptic cell, whereas, at a weak synapse, the excitatory postsynaptic potential ("EPSP") will not reach the threshold for action potential initiation. In the brain, however, each neuron forms synapses with many others, and, likewise, each receives synaptic inputs from many others. When action potentials fire simultaneously in several neurons that weakly synapse on a single cell, they may initiate an impulse in that cell even though the synapses are weak. This process is known as summation. On the other hand, a presynaptic neuron releasing an inhibitory neurotransmitter such as GABA can cause inhibitory postsynaptic potential in the postsynaptic neuron, decreasing its excitability and therefore decreasing the neuron's likelihood of firing an action potential. In this way, the output of a neuron may depend on the input of many others, each of which may have a different degree of influence, depending on the strength of its synapse with that neuron. John Carew Eccles performed some of the important early experiments on synaptic integration, for which he received the Nobel Prize for Physiology or Medicine in 1963. Complex input/output relationships form the basis of transistor-based computations in computers, and are thought to figure similarly in neural circuits.
Synaptic strength
The strength of a synapse is defined by the change in transmembrane potential resulting from activation of the postsynaptic neurotransmitter receptors. This change in voltage is known as a postsynaptic potential, and is a direct result of ionic currents flowing through the postsynaptic ion channels. Changes in synaptic strength can be short–term and without permanent structural changes in the neurons themselves, lasting seconds to minutes — or long-term ( long-term potentiation, or LTP), in which repeated or continuous synaptic activation can result in second messenger molecules initiating protein synthesis, resulting in alteration of the structure of the synapse itself. Learning and memory are believed to result from long-term changes in synaptic strength, via a mechanism known as synaptic plasticity.
Relationship to electrical synapses
An electrical synapse is a mechanical and electrically conductive link between two abutting neurons that is formed at a narrow gap between the pre- and postsynaptic cells known as a gap junction. At gap junctions, cells approach within about 3.5 nm of each other, rather than the 20 to 40 nm distance that separates cells at chemical synapses. As opposed to chemical synapses, the postsynaptic potential in electrical synapses is not caused by the opening of ion channels by chemical transmitters, but by direct electrical coupling between both neurons. Electrical synapses are therefore faster and more reliable than chemical synapses. Electrical synapses are found throughout the nervous system, yet are less common than chemical synapses.