Yellow fever
2008/9 Schools Wikipedia Selection. Related subjects: Health and medicine
| Yellow fever Classification and external resources |
|
| ICD- 10 | A 95. |
|---|---|
| ICD- 9 | 060 |
| DiseasesDB | 14203 |
| MedlinePlus | 001365 |
| eMedicine | med/2432 emerg/645 |
| MeSH | D015004 |
Yellow fever (also called yellow jack, black vomit or sometimes American Plague) is an acute viral disease. It is an important cause of hemorrhagic illness in many African and South American countries despite existence of an effective vaccine. The yellow refers to the jaundice symptoms that affect some patients.
Yellow fever has been a source of several devastating epidemics. French soldiers were attacked by yellow fever during the 1802 Haitian Revolution; more than half of the army perished from the disease. Outbreaks followed by thousands of deaths occurred periodically in other Western Hemisphere locations until research, which included human volunteers (some of whom died), led to an understanding of the method of transmission to humans (primarily by mosquitos) and development of a vaccine and other preventive efforts in the early 20th century.
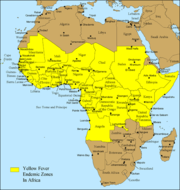
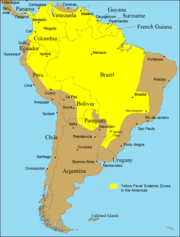
Despite the costly and sacrificial breakthrough research by Cuban physician Carlos Finlay, American physician Walter Reed, and many others over 100 years ago, unvaccinated populations in many developing nations in Africa and Central and South America continue to be at risk. As of 2001, the World Health Organization (WHO) estimates that yellow fever causes 200,000 illnesses and 30,000 deaths every year in unvaccinated populations.
Pathogenesis
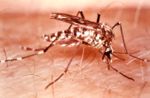
Yellow fever is caused by an arbovirus of the family Flaviviridae, a positive sense single-stranded RNA virus. Human infection begins after deposition of viral particles through the skin in infected arthropod saliva. The mosquitos involved are Aedes simpsaloni, A. africanus, and A. aegypti in Africa, the Haemagogus genus in South America, and the Sabethes genus in France. Yellow fever is frequently severe but moderate cases may occur as the result of previous infection by another flavivirus. After infection the virus first replicates locally, followed by transportation to the rest of the body via the lymphatic system. Following systemic lymphatic infection the virus proceeds to establish itself throughout organ systems, including the heart, kidneys, adrenal glands, and the parenchyma of the liver; high viral loads are also present in the blood. Necrotic masses ( Councilman bodies) appear in the cytoplasm of hepatocytes.,
Yellow fever begins suddenly after an incubation period of three to five days in the human body. In mild cases only fever and headache may be present. The severe form of the disease commences with fever, chills, bleeding into the skin, rapid heartbeat, headache, back pains, and extreme prostration. Nausea, vomiting, and constipation are common. Jaundice usually appears on the second or third day. After the third day the symptoms recede, only to return with increased severity in the final stage, during which there is a marked tendency to hemorrhage internally; the characteristic “coffee ground” vomitus contains blood. The patient then lapses into delirium and coma, often followed by death. During epidemics the fatality rate was often as high as 85%. Although the disease still occurs, it is usually confined to sporadic outbreaks.
There is a difference between disease outbreaks in rural or forest areas and in towns. Disease outbreaks in towns and non-native people may be more serious because of higher densities of mosquito vectors and higher population densities.
Prevention
In 1937, Max Theiler, working at the Rockefeller Foundation, developed a safe and highly efficacious vaccine for yellow fever that gives a ten-year or more immunity from the virus. The vaccine consists of a live, but attenuated, virus called 17D. The 17D vaccine has been used commercially since the 1950s. The mechanisms of attenuation and immunogenicity for the 17D strain are not known. However, this vaccine is very safe, with few adverse reactions having been reported and millions of doses administered, and highly effective with over 90% of vaccinees developing a measurable immune response after the first dose.
Although the vaccine is considered safe, there are risks involved. The majority of adverse reactions to the 17D vaccine result from allergic reaction to the eggs in which the vaccine is grown. Persons with a known egg allergy should discuss this with their physician prior to vaccination. In addition, there is a small risk of neurologic disease and encephalitis, particularly in individuals with compromised immune systems and very young children. The 17D vaccine is contraindicated in infants, pregnant women, and anyone with a diminished immune capacity, including those taking immunosuppressant drugs.
According to the travel clinic at the University of Utah Hospital, the vaccine presents an increased risk of adverse reaction in adults aged 60 and older, with the risk increasing again after age 65, and again after age 70. The reaction is capable of producing multiple organ failure and should be evaluated carefully by a qualified health professional before being administered to the elderly.
Finally, there is a very small risk of more severe yellow fever-like disease associated with the vaccine. This reaction occurs in ~1-3 vaccinees per million doses administered. This reaction, called YEL-AVD, causes a fairly severe disease closely resembling yellow fever caused by virulent strains of the virus. The risk factor/s for YEL-AVD are not known, although it has been suggested that it may be genetic. The 2`-5` oligoadenylate synthetase (OAS) component of the innate immune response has been shown to be particularly important in protection from Flavivirus infection. In at least one case of YEL-AVD, the patient was found to have an allelic mutation in a single nucleotide polymorphism (SNP) of the OAS gene. People most at risk of contracting the virus should be vaccinated. Woodcutters working in tropical areas should be particularly targeted for vaccination. Insecticides, protective clothing, and screening of houses are helpful, but not always sufficient for mosquito control; people should always use an insecticide spray while in certain areas. In affected areas, mosquito control methods have proven effective in decreasing the number of cases.
Recent studies have noted the increase in the number of areas affected by a number of mosquito-borne viral infections and have called for further research and funding for vaccines.,
Treatment
There is no true cure for yellow fever, therefore vaccination is important. Treatment is symptomatic and supportive only. Fluid replacement, fighting hypotension and transfusion of blood derivates is generally needed only in severe cases. In cases that result in acute renal failure, dialysis may be necessary.
Current research
In the hamster model of yellow fever, early administration of the antiviral ribavirin is an effective early treatment of many pathological features of the disease. Ribavirin treatment during the first five days after virus infection improved survival rates, reduced tissue damage in target organs (liver and spleen), prevented hepatocellular steatosis, and normalized alanine aminotransferase (a liver damage marker) levels. The results of this study suggest that ribavirin may be effective in the early treatment of yellow fever, and that its mechanism of action in reducing liver pathology in yellow fever virus infection may be similar to that observed with ribavirin in the treatment of hepatitis C, a virus related to yellow fever. Because ribavirin had failed to improve survival in a virulent primate (rhesus) model of yellow fever infection, it had been previously discounted as a possible therapy.
In 2007, the World Community Grid launched a project where by computer modeling of the yellow fever virus (and related viruses) thousands of small molecules are screened for their potential anti-viral properties in fighting yellow fever. This is the first project to utilize computer simulations in seeking out medicines to directly attack the virus once a person is infected. This is a distributed process project similar to SETI@Home where the general public downloads the World Community Grid agent and the program (along with thousands of other users) screens thousands of molecules while their computer would be otherwise idle. If the user needs to use the computer the program sleeps. There are several different projects running, including a similar one screening for anti-AIDS drugs. The project covering yellow fever is called "Discovering Dengue Drugs – Together." The software and information about the project can be found at:
- World Community Grid web site
Prognosis
Historical reports have claimed a mortality rate of between 1 in 17 (5.8%) and 1 in 3 (33%). CDC has claimed that case-fatality rates from severe disease range from 15% to more than 50%. The WHO factsheet on yellow fever, updated in 2001, states that 15% of patients enter a "toxic phase" and that half of that number die within ten to fourteen days, with the other half recovering.
History
Yellow fever has had an important role in the history of Africa, the Americas, Europe, and the Caribbean.
Europe: 541-549
Fragile after the fall of Rome, Europe was further weakened by "Yellow Plague" (yellow fever). The Byzantine Empire suffered as well.
Cuba: 1762-1763
British and American colonial troops died by the thousands in the British expedition against Cuba between 1762 and 1763. Epidemics struck coastal and island communities throughout the area during the next 140 years, with 10% of the population dying as a result.
Haiti: 1802
In 1802, an army of forty thousand sent by First Consul Napoleon Bonaparte of France to Haiti to suppress the Haitian Revolution was dwindled out by an epidemic of yellow fever (including the expedition's commander and Bonaparte's brother-in-law, Charles Leclerc). Some historians believe Haiti was to be a staging point for an invasion of the United States through Louisiana (then still under French control).
Norfolk, Virginia: 1855
A ship carrying persons infected with the virus arrived in Hampton Roads in southeastern Virginia in June 1855 . The disease spread quickly through the community, eventually killing over 3,000 people, mostly residents of Norfolk and Portsmouth. The Howard Association, a benevolent organization, was formed to help coordinate assistance in the form of funds, supplies, and medical professionals and volunteers which poured in from many other areas, particularly the Atlantic and Gulf Coast areas of the United States. See also The Mermaids and Yellow Jack: A NorFolktale a children's historical fiction written by Norfolk author Lisa Suhay, telling of the event and founding of the Bon Secours DePaul Hospital system in the United States in response to the epidemic.
Memphis, Tennessee: 1878
The worst yellow fever epidemic in U.S. history occurred in 1878, with over 5,000 deaths in Memphis alone and 20,000 deaths in the whole of the Mississippi Valley.
Carlos Finlay and Walter Reed
Carlos Finlay, a Cuban doctor and scientist, first proposed proofs in 1881 that yellow fever is transmitted by mosquitoes rather than direct human contact. Walter Reed, M.D., (1851-1902) was an American Army surgeon who led a team that confirmed Finlay's theory. This risky but fruitful research work was done with human volunteers, including some of the medical personnel such as Clara Maass and Walter Reed Medal winner surgeon Jesse William Lazear who allowed themselves to be deliberately infected and died of the virus. The acceptance of Finlay's work was one of the most important and far-reaching effects of the Walter Reed Commission of 1900. Applying methods first suggested by Finlay, the elimination of yellow fever from Cuba was completed, as well as the completion of the Panama Canal. Lamentably, almost 20 years had passed before Reed and his Board began their efforts, twenty years during which most of the scientific community ignored Finlay's theory of mosquito transmission.
Finlay and Reed's work was put to the test for the first time in the United States when a yellow fever epidemic struck New Orleans in 1905, although efforts had been successful in Havana since 1901; according to the PBS American Experience documentary The Great Fever, houses were fumigated, cisterns for drinking water were inspected, and pools of standing water were treated with kerosene. The result was that the death toll from the epidemic was much lower than that from previous yellow fever epidemics, and that there has not been a major outbreak of the disease in the United States since. Although no cure has yet been discovered, an effective vaccine has been developed, which can prevent and help people recover from the disease.