J. J. Thomson
2008/9 Schools Wikipedia Selection. Related subjects: British History Post 1900; Engineers and inventors
| J. J. Thomson | |
 Sir Joseph John Thomson (1856-1940). Portrait by Arthur Hacker.
|
|
| Born | 18 December 1856 Cheetham Hill, Manchester, UK |
|---|---|
| Died | 30 August 1940 (aged 83) Cambridge, UK |
| Nationality | United Kingdom |
| Fields | Physicist |
| Institutions | University of Cambridge |
| Alma mater | University of Manchester University of Cambridge |
| Academic advisors | John Strutt (Rayleigh) Edward John Routh |
| Notable students | Charles T. R. Wilson Ernest Rutherford Francis William Aston John Townsend J. Robert Oppenheimer Owen Richardson William Henry Bragg H. Stanley Allen John Zeleny Daniel Frost Comstock Max Born T. H. Laby Paul Langevin |
| Known for | Plum pudding model Discovery of electron Discovery of isotopes Mass spectrometer invention First m/e measurement Proposed first waveguide Thomson scattering Thomson problem Coining term 'delta ray' Coining term 'epsilon radiation' Thomson (unit) |
| Notable awards | Nobel Prize for Physics (1906) |
| Religious stance | Anglican |
Signature |
|
|
Notes
Thomson is the father of Nobel laureate George Paget Thomson. |
|
Sir Joseph John “J.J.” Thomson, OM, FRS ( 18 December 1856 – 30 August 1940) was a British physicist and Nobel laureate, credited for the discovery of the electron and of isotopes, and the invention of the mass spectrometer. He was awarded the 1906 Nobel Prize in Physics for the discovery of the electron and his work on the conduction of electricity in gases.
Biography
J.J. Thomson was born in 1856 in Cheetham Hill, Manchester in England, of Scottish parentage. In 1870 he studied engineering at University of Manchester known as Owens College at that time, and moved on to Trinity College, Cambridge in 1876. In 1880, he obtained his BA in mathematics ( Second Wrangler and 2nd Smith's prize) and MA (with Adams Prize) in 1883. In 1884 he became Cavendish Professor of Physics. One of his students was Ernest Rutherford, who would later succeed him in the post. In 1890 he married Rose Elisabeth Paget, daughter of Sir George Edward Paget, KCB, a physician and then Regius Professor of Physic at Cambridge. He fathered one son, George Paget Thomson, and one daughter, Joan Paget Thomson, with her. One of Thomson's greatest contributions to modern science was in his role as a highly gifted teacher, as seven of his research assistants and his aforementioned son won Nobel Prizes in physics. His son won the Nobel Prize in 1937 for proving the wavelike properties of electrons.
He was awarded a Nobel Prize in 1906, "in recognition of the great merits of his theoretical and experimental investigations on the conduction of electricity by gases." He was knighted in 1908 and appointed to the Order of Merit in 1912. In 1914 he gave the Romanes Lecture in Oxford on "The atomic theory". In 1918 he became Master of Trinity College, Cambridge, where he remained until his death. He died on August 30, 1940 and was buried in Westminster Abbey, close to Sir Isaac Newton.
Thomson was elected a Fellow of the Royal Society on June 12, 1884 and was subsequently President of the Royal Society from 1915 to 1920.
Career
Cathode rays
Thomson conducted a series of experiments with cathode rays and cathode ray tubes leading him to the discovery of electrons and subatomic particles. Thomson used the cathode ray tube in three different experiments.
First experiment
In his first experiment, he investigated whether or not the negative charge could be separated from the cathode rays by means of magnetism. He constructed a cathode ray tube ending in a pair of cylinders with slits in them. These slits were in turn connected to an electrometer. Thomson found that if the rays were magnetically bent such that they could not enter the slit, the electrometer registered little charge. Thomson concluded that the negative charge was inseparable from the rays.
Second experiment
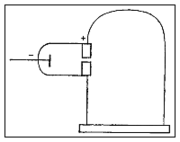
In his second experiment, he investigated whether or not the rays could be deflected by an electric field (something that is characteristic of charged particles). Previous experimenters had failed to observe this, but Thomson believed their experiments were flawed because they contained trace amounts of gas. Thomson constructed a cathode ray tube with a practically perfect vacuum, and coated one end with phosphorescent paint. Thomson found that the rays did indeed bend under the influence of an electric field, in a direction indicating a negative charge.
Third experiment
In his third experiment, Thomson measured the charge-to-mass ratio of the cathode rays by measuring how much they were deflected by a magnetic field and how much energy they carried. He found that the charge to mass ratio was over a thousand times higher than that of a hydrogen ion (H+), suggesting either that the particles were very light or very highly charged.
Thomson's conclusions were bold: cathode rays were indeed made of particles which he called " corpuscles", and these corpuscles came from within the atoms of the electrodes themselves, meaning that atoms are in fact divisible. The "corpuscles" discovered by Thomson are identified with the electrons which had been proposed by G. Johnstone Stoney.
Thomson imagined the atom as being made up of these corpuscles swarming in a sea of positive charge; this was his plum pudding model. This model was later proved incorrect when Ernest Rutherford showed that the positive charge is concentrated in the nucleus.
Thomson's discovery was made known in 1897, and caused a sensation in scientific circles, eventually resulting in him being awarded a Nobel Prize in Physics in 1906.
Isotopes and mass spectrometry
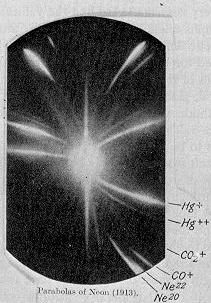
In 1913, as part of his exploration into the composition of canal rays, Thomson channelled a stream of ionized neon through a magnetic and an electric field and measured its deflection by placing a photographic plate in its path. Thomson observed two patches of light on the photographic plate (see image on right), which suggested two different parabolas of deflection. Thomson concluded that the neon gas was composed of atoms of two different atomic masses (neon-20 and neon-22).
This separation of neon isotopes by their mass was the first example of mass spectrometry, which was subsequently improved and developed into a general method by Thomson's student F. W. Aston and by A. J. Dempster.
Other work
In 1906 Thomson demonstrated that hydrogen had only a single electron per atom. Previous theories allowed various numbers of electrons.
Awards
- Royal Medal (1894)
- Hughes Medal (1902)
- Nobel Prize for Physics (1906)
- Copley Medal (1914)