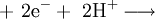Electrolysis
2008/9 Schools Wikipedia Selection. Related subjects: Chemistry
In chemistry and manufacturing, electrolysis is a method of separating chemically bonded elements and compounds by passing an electric current through them.
Overviews
Electrolysis involves the passage of an electric current through, in general, an ionic substance that is either molten or dissolved in an aqueous solution resulting in chemical reactions at the electrodes. The negative electrode is called the cathode, and the positive electrode is the anode.
An ionic compound is dissolved with an appropriate solvent, or melted by heat, so that its ions are available in the liquid. An electrical current is applied between a pair of inert electrodes immersed in the liquid. Each electrode attracts ions that are of the opposite charge. Therefore, positively-charged ions (called cations) move towards the cathode, whereas negatively-charged ions (termed anions) move toward the anode. The energy required to separate the ions, and cause them to gather at the respective electrodes, is provided by an electrical power supply. At the probes, electrons are absorbed or released by the ions, forming a collection of the desired element or compound.
Oxidation of anions can take place at the anode, and the reduction of cations at the cathode. For example, it is possible to oxidize cations at the anode:
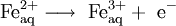 .
.
It is also possible to reduce anions at the cathode:
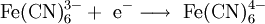 .
.
Neutral molecules can also react at either electrode. For example:
The amount of electrical energy that must be added equals the change in Gibbs free energy of the reaction plus the losses in the system. The losses can (in theory) be arbitrarily close to zero, so the maximum thermodynamic efficiency equals the enthalpy change divided by the free energy change of the reaction. In most cases, the electric input is larger than the enthalpy change of the reaction, so some energy is released in the form of heat. In some cases, for instance, in the electrolysis of steam into hydrogen and oxygen at high temperature, the opposite is true. Heat is absorbed from the surroundings, and the heating value of the produced hydrogen is higher than the electric input. (It is worth noting that the maximum theoretic efficiency of a fuel cell is the inverse of that of electrolysis. It is, thus, impossible to create a perpetual motion machine by combining the two processes. See water fuel cell for an example of such an attempt.)
The following technologies are related to electrolysis:
- Electrochemical cells, including the hydrogen fuel cell, use the reverse of this process.
- Gel electrophoresis is an electrolysis wherein the solvent is a gel: It is used to separate substances, such as DNA strands, based on their electrical charge.
Electrolysis of water
One important use of electrolysis of water is to produce hydrogen.
- 2H2O(l) → 2H2(g) + O2(g)
This has been suggested as a way of shifting society toward using hydrogen as an energy carrier for powering electric motors and internal combustion engines. (See hydrogen economy.)
Electrolysis of water can be observed by passing direct current from a battery or other DC power supply through a cup of water (in practice a saltwater solution increases the reaction intensity making it easier to observe). Using platinum electrodes, hydrogen gas will be seen to bubble up at the cathode, and oxygen will bubble at the anode. If other metals are used as the anode, there is a chance that the oxygen will react with the anode instead of being released as a gas. For example, using iron electrodes in a sodium chloride solution electrolyte, iron oxide will be produced at the anode, which will react to form iron hydroxide. When producing large quantities of hydrogen, this can significantly contaminate the electrolytic cell - which is why iron is not used for commercial electrolysis.
The energy efficiency of water electrolysis varies widely. The efficiency is a measure of what fraction of electrical energy used is actually contained within the hydrogen. Some of the electrical energy is converted to heat, a useless by-product. Some reports quote efficiencies between 50% and 70% This efficiency is based on the Lower Heating Value of Hydrogen. The Lower Heating Value of Hydrogen is thermal energy released when Hydrogen is combusted. This does not represent the total amount of energy within the Hydrogen, hence the efficiency is lower than a more strict definition. Other reports quote the theoretical maximum efficiency of electrolysis as being between 80% and 94%. . The theoretical maximum considers the total amount of energy absorbed by both the hydrogen and oxygen. These values refer only to the efficiency of converting electrical energy into hydrogen's chemical energy. The energy lost in generating the electricity is not included. For instance, when considering a power plant that converts the heat of nuclear reactions into hydrogen via electrolysis, the total efficiency is more like 25%–40%.
About four percent of hydrogen gas produced worldwide is created by electrolysis, and normally used onsite. Hydrogen is used for the creation of ammonia for fertilizer via the Haber process, and converting heavy petroleum sources to lighter fractions via hydrocracking.
Experimenters
Scientific pioneers of electrolysis included:
- Humphry Davy
- Michael Faraday
- Paul Héroult
- Svante Arrhenius
- Adolph Wilhelm Hermann Kolbe
- William Nicholson
- Joseph Louis Gay-Lussac
- Alexander von Humboldt
More recently, electrolysis of heavy water was performed by Fleischmann and Pons in their famous experiment, allegedly resulting in anomalous heat generation and the controversial claim of cold fusion.
Faraday's laws of electrolysis
First law of electrolysis
In 1832, Michael Faraday reported that the quantity of elements separated by passing an electrical current through a molten or dissolved salt is proportional to the quantity of electric charge passed through the circuit. This became the basis of the first law of electrolysis:
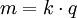
Second law of electrolysis
Faraday also discovered that the mass of the resulting separated elements is directly proportional to the atomic masses of the elements when an appropriate integral divisor is applied. This provided strong evidence that discrete particles of matter exist as parts of the atoms of elements.
Industrial uses
- Production of aluminium, lithium, sodium, potassium
- Production of hydrogen for hydrogen cars and fuel cells; high-temperature electrolysis is also used for this
- Coulometric techniques can be used to determine the amount of matter transformed during electrolysis by measuring the amount of electricity required to perform the electrolysis
- Production of chlorine and sodium hydroxide
- Production of sodium and potassium chlorate
- Production of perfluorinated organic compounds such as trifluoroacetic acid
Electrolysis has many other uses:
- Electrometallurgy is the process of reduction of metals from metallic compounds to obtain the pure form of metal using electrolysis. For example, sodium hydroxide in its molten form is separated by electrolysis into sodium and oxygen, both of which have important chemical uses. (Water is produced at the same time.)
- Anodization is an electrolytic process that makes the surface of metals resistant to corrosion. For example, ships are saved from being corroded by oxygen in the water by this process. The process is also used to decorate surfaces.
- A battery works by the reverse process to electrolysis. Humphry Davy found that lithium acts as an electrolyte and provides electrical energy.
- Production of oxygen for spacecraft and nuclear submarines.
- Electroplating is used in layering metals to fortify them. Electroplating is used in many industries for functional or decorative purposes, as in vehicle bodies and nickel coins.
- Production of hydrogen for fuel, using a cheap source of electrical energy.
- Electrolytic Etching of metal surfaces like tools or knives with a permanent mark or logo.
Electrolysis is also used in the cleaning and preservation of old artifacts. Because the process separates the non-metallic particles from the metallic ones, it is very useful for cleaning old coins and even larger objects.